Organocatalytic Asymmetric Synthesis of Dihydroisoquinolinones via a One-Pot Aza-Henry-Hemiaminalization-Oxidation Sequence
- PMID: 26722132
- PMCID: PMC4693956
- DOI: 10.1055/s-0034-1379398
Organocatalytic Asymmetric Synthesis of Dihydroisoquinolinones via a One-Pot Aza-Henry-Hemiaminalization-Oxidation Sequence
Abstract
The asymmetric organocatalytic one-pot synthesis of trans-3,4-disubstituted 3,4-dihydroisoquinolin-1(2H)-ones is described. Starting from 2-(nitromethyl)benzaldehydes and various N-protected aldimines, 5 mol% of a quinine-based squaramide organocatalyst was used to synthesize the title compounds as virtually single diastereomers via an aza-Henry-hemiaminalization-oxidation sequence. Moderate to good yields (39-78%) and moderate to very good enantioselectivities (40-95% ee) were reached.
Keywords: dihydroisoquinolinones; domino reaction; hydrogen bonding; one-pot reaction; organocatalysis.
Figures

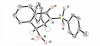

Similar articles
-
Asymmetric synthesis of tetrahydropyridines via an organocatalytic one-pot multicomponent Michael/aza-Henry/cyclization triple domino reaction.Org Lett. 2014 Nov 21;16(22):6012-5. doi: 10.1021/ol503024d. Epub 2014 Nov 7. Org Lett. 2014. PMID: 25379786 Free PMC article.
-
Asymmetric synthesis of highly functionalized tetrahydropyrans via a one-pot organocatalytic Michael/Henry/ketalization sequence.Org Lett. 2014 Jul 18;16(14):3636-9. doi: 10.1021/ol501236a. Epub 2014 Jun 27. Org Lett. 2014. PMID: 24971998 Free PMC article.
-
Asymmetric Synthesis of Functionalized Dihydro- and Tetrahydropyrans via an Organocatalytic Domino Michael-Hemiacetalization Reaction.Synthesis (Stuttg). 2014 May 1;46(9):1261-1269. doi: 10.1055/s-0033-1340826. Synthesis (Stuttg). 2014. PMID: 25284900 Free PMC article.
-
[Development of Green Asymmetric Organocatalytic Synthesis].Yakugaku Zasshi. 2021;141(10):1137-1145. doi: 10.1248/yakushi.21-00130. Yakugaku Zasshi. 2021. PMID: 34602510 Review. Japanese.
-
Squaramide-Catalyzed Asymmetric Reactions.Chem Rec. 2017 Oct;17(10):994-1018. doi: 10.1002/tcr.201600140. Epub 2017 Mar 7. Chem Rec. 2017. PMID: 28266131 Review.
Cited by
-
Traceless Redox-Annulations of Alicyclic Amines.SynOpen. 2020;4(4):123-131. doi: 10.1055/s-0040-1706004. Epub 2020 Dec 16. SynOpen. 2020. PMID: 34327302 Free PMC article.
-
Development of a Cross-Conjugated Vinylogous [4+2] Anionic Annulation and Application to the Total Synthesis of Natural Antibiotic (±)-ABX.Angew Chem Int Ed Engl. 2020 Apr 16;59(16):6540-6545. doi: 10.1002/anie.201914657. Epub 2020 Feb 25. Angew Chem Int Ed Engl. 2020. PMID: 31944523 Free PMC article.
References
-
- Shamma M. Isoquinoline Alkaloids, Chemistry and Pharmacology. Academic; New York: 1972.
- Müjde B, Özcan S, Balci M. Phytochemistry Lett. 2011;4:407.
-
-
For the isolation of thalflavine, see:
- Umarov KS, Ismailov ZF, Yunusov SY. Chem. Nat. Compd. 1970;6:452.
- Popović M, Djurković R, Gasić O, Pal B, Dutschewska H, Kuzmanov B. Biochem. Syst. Ecol. 1992;20:255.
-
For the revision of the proposed structure, see:
- Aly Y, Galal A, Wong LK, Fu EW, Lin F-T, Duah FK, Schiff PL., Jr. Phytochemistry. 1989;28:1967.
-
-
-
For the isolation of pancratistatin, see:
- Pettit GR, Gaddamidi V, Cragg GM, Herald DL, Sagawa Y. J. Chem. Soc., Chem. Commun. 1984:1693.
- Pettit GR, Gaddamidi V, Cragg GM. J. Nat. Prod. 1984;47:1018. - PubMed
- Pettit GR, Gaddamidi V, Herald DL, Singh SB, Cragg GM, Schmidt JM, Boettner FE, Williams M, Sagawa Y. J. Nat. Prod. 1986;49:995. - PubMed
-
For selected total syntheses, see:
- Danishefsky S, Lee JY. J. Am. Chem. Soc. 1989;111:4829.
- Tian X, Hudlicky T, Königsberger K. J. Am. Chem. Soc. 1995;117:3643.
- Rigby JH, Maharoof USM, Mateo ME. J. Am. Chem. Soc. 2000;122:6624.
- Ko HJ, Kim E, Park JE, Kim D, Kim S. J. Org. Chem. 2004;69:112. - PubMed
- Dam JH, Madsen R. Eur. J. Org. Chem. 2009:4666.
- Jung Y-G, Kang H-U, Cho H-K, Cho C-G. Org. Lett. 2011;13:5890. - PubMed
-
For selected references on the biological activity, see:
- Pettit GR, Pettit GR, III, Backhaus RA, Boyd MR, Meerow AW. J. Nat. Prod. 1993;56:1682. - PubMed
- Gabrielsen B, Montath TP, Huggins JW, Kefauver DF, Pettit GR, Groszek G, Hollingshead M, Kirsi JJ, Shannon WM, Schubert EM, Dare J, Ugarkar B, Ussery MA, Phelan MJ. J. Nat. Prod. 1992;55:1569. - PubMed
-
-
-
For the isolation of plicamine, see:
- Ünver N, Gözler T, Walch N, Gözler B, Hesse M. Phytochemistry. 1999;50:1255.
-
For reported total syntheses, see:
- Baxendale IR, Ley SV, Piutti C. Angew. Chem. Int. Ed. 2002;41:2194. - PubMed
- Baxendale IR, Ley SV, Nessi M, Piutti C. Tetrahedron. 2002;58:6285.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
