Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis
- PMID: 26722385
- PMCID: PMC4680330
Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis
Abstract
Objective: To investigate the roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis.
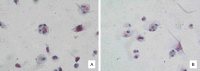
Methods: Diabetic rat model was established; tissue section technology was used to observe the situation of osteoporosis in diabetic rats; rat serum levels of OC, RANKL, GSK-3β, P38mapk, TNF-α and INS were detected by Elisa assay; osteoblasts and osteoclasts were primarily cultured and identified by immunohistochemistry and tartrate-resistant acid phosphatase (TRAP) staining respectively. The effects of GSK-3β inhibitors, lithium chloride, TNF-α antagonists and RANKL antagonists on the proliferation of osteoblasts and osteoclasts were evaluated; quantitative PCR was used to assess the effects of GSK-3β inhibitors, lithium chloride, on TNF-α and RANKL gene expression in osteoblasts and osteoclasts, and the effects of TNF-α and RANKL antagonists on GSK-3β gene expression in osteoblasts and osteoclasts.
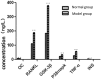
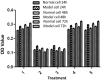
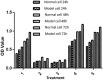
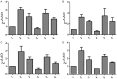
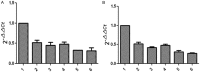
Results: Diabetic rat model was successfully established; osteoblasts and osteoclasts were successfully isolated and cultured. Elisa experiments showed that in diabetic model group, the levels of RANKL, GSK-3β, P38mapk and TNF-α were significantly increased, while the levels of osteocalcin (OC) and insulin (INS) were significantly reduced; MTT results showed that osteoclast proliferation in GSK-3β inhibitor and lithium chloride groups were weaker than the untreated group, while osteoclast proliferation in TNF-α antagonist group and RANKL antagonist Group was very close to the untreated group. Osteoblast proliferation in GSK-3β inhibitor and lithium chloride groups were weaker than the untreated group, while osteoblast proliferation in TNF-α antagonist group and RANKL antagonist group was higher than the untreated group. In all of the corresponding groups, cell proliferation in the diabetic group was stronger than the untreated group. In GSK-3β inhibitor and lithium oxide groups, TNF-α and RANKL gene expression levels were elevated, but TNF-α and RANKL gene expression levels in the diabetic group were slightly lower than the control group. GSK-3β gene expression level in TNF-α antagonist group and RANKL antagonist group was reduced; GSK-3β gene expression level in diabetic group was lower than the control group.
Conclusion: In diabetic rats, TNF-α, GSK-3β and RANKL levels were elevated; GSK-3β could promote the proliferation of osteoblasts and osteoclasts, and inhibit the expression of TNF-α and RANKL; TNF-α and RANKL can suppress the proliferation of osteoblasts while had little effect on osteoclast proliferation; they also can promote the GSK-3β gene expression; interactions between the three broke the balance between osteoblasts and osteoclasts, leading to osteoporosis.
Keywords: GSK-3β; RANKL; TNF-α; diabetes; osteoporosis.
Figures



Similar articles
-
GSK-3β inhibition suppresses instability-induced osteolysis by a dual action on osteoblast and osteoclast differentiation.J Cell Physiol. 2018 Mar;233(3):2398-2408. doi: 10.1002/jcp.26111. Epub 2017 Sep 28. J Cell Physiol. 2018. PMID: 28731198 Free PMC article.
-
Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse.J Bone Miner Res. 2005 Sep;20(9):1659-68. doi: 10.1359/JBMR.050503. Epub 2005 May 2. J Bone Miner Res. 2005. PMID: 16059637
-
[Effect of GSK-3β inhibitor on the expression of RANK-RANKL in rats kidney tissue with diabetic nephropathy].Zhonghua Bing Li Xue Za Zhi. 2018 Dec 8;47(12):945-950. doi: 10.3760/cma.j.issn.0529-5807.2018.12.010. Zhonghua Bing Li Xue Za Zhi. 2018. PMID: 30522177 Chinese.
-
Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover.Int J Mol Sci. 2022 Jan 25;23(3):1376. doi: 10.3390/ijms23031376. Int J Mol Sci. 2022. PMID: 35163300 Free PMC article. Review.
-
[Hormones and osteoporosis update. Effect of angiotensin II on bone metabolism].Clin Calcium. 2009 Jul;19(7):997-1002. Clin Calcium. 2009. PMID: 19567997 Review. Japanese.
Cited by
-
Zhuang-Gu-Fang intervenes vasculogenic and osteogenic coupling in GK rats through Notch1/Noggin/VEGF pathway.Heliyon. 2024 Mar 13;10(6):e28014. doi: 10.1016/j.heliyon.2024.e28014. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524608 Free PMC article.
-
Recent Advances of Osterix Transcription Factor in Osteoblast Differentiation and Bone Formation.Front Cell Dev Biol. 2020 Dec 15;8:601224. doi: 10.3389/fcell.2020.601224. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33384998 Free PMC article. Review.
-
Glycogen Synthase Kinase-3 Beta (GSK3β) as a Potential Drug Target in Regulating Osteoclastogenesis: An Updated Review on Current Evidence.Biomolecules. 2024 Apr 21;14(4):502. doi: 10.3390/biom14040502. Biomolecules. 2024. PMID: 38672518 Free PMC article. Review.
-
Lithium chloride stimulates bone formation in extraction socket repair in rats.Oral Maxillofac Surg. 2024 Mar;28(1):169-177. doi: 10.1007/s10006-022-01124-4. Epub 2022 Oct 15. Oral Maxillofac Surg. 2024. PMID: 36242702
-
Hops extract and xanthohumol ameliorate bone loss induced by iron overload via activating Akt/GSK3β/Nrf2 pathway.J Bone Miner Metab. 2022 May;40(3):375-388. doi: 10.1007/s00774-021-01295-2. Epub 2022 Feb 1. J Bone Miner Metab. 2022. PMID: 35106609
References
-
- Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castaño-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hofman A, van Leeuwen JP, Witteman JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care. 2013;36:1619–1628. - PMC - PubMed
-
- Mazess RB. Fracture risk: a role for compact bone. Calcif Tissue Int. 1990;47:191–193. - PubMed
-
- Riggs BL, Khosla S, Melton LJ 3rd. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. - PubMed
-
- Doherty RO, Stein D, Foley J. Insulin resistance. Diabetologia. 1997;40:B10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
