Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs
- PMID: 26724471
- PMCID: PMC4979601
- DOI: 10.1016/j.jtho.2015.12.103
Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs
Abstract
Introduction: Activating mutations in the epidermal growth factor receptor gene (EGFR) predict for prolonged progression-free survival in patients with advanced non-small cell lung cancer (NSCLC) treated with EGFR tyrosine kinase inhibitors (EGFR-TKIs) versus chemotherapy. Long-term survival outcomes, however, remain undefined. The objective of this study was to determine the 5-year survival in these patients and identify clinical factors associated with overall survival (OS).
Methods: Patients with EGFR-mutant metastatic lung adenocarcinoma who had been treated with erlotinib or gefitinib at Dana-Farber Cancer Institute between 2002 and 2009 were included. OS was analyzed.
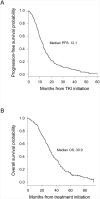
Results: Among 137 patients, median progression-free survival and OS were 12.1 months (95% CI: 10.2-13.5) and 30.9 months (95% CI: 28.2-35.7), respectively. Twenty patients (14.6%) were 5-year survivors. In multivariate analysis, exon 19 deletions (hazard ratio [HR] = 0.63, 95% CI: 0.44-0.91, p = 0.01), absence of extrathoracic (HR = 0.62, 95% CI: 0.41-0.93, p = 0.02) or brain metastasis (HR = 0.48, 95% CI: 0.30-0.77, p = 0.002), and not a current smoker (HR = 0.23, 95% CI: 0.09-0.59, p = 0.002) were associated with prolonged OS. Age; sex; stage at diagnosis; liver, bone, or adrenal metastasis; specific TKI; and line of TKI therapy were not associated with OS.
Conclusions: Our data suggest that the rate of 5-year survival among patients with EGFR-mutant metastatic lung adenocarcinoma treated with erlotinib or gefitinib is 14.6%. Exon 19 deletions and absence of extrathoracic or brain metastasis are associated with prolonged survival. On the basis of our findings, clinicians can gain an enhanced estimation of long-term outcomes in this population.
Keywords: EGFR; Non–small cell lung cancer; TKI; long-term survival.
Copyright © 2015 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Metastatic lung cancer in the age of targeted therapy: improving long-term survival.Transl Lung Cancer Res. 2016 Dec;5(6):727-730. doi: 10.21037/tlcr.2016.11.08. Transl Lung Cancer Res. 2016. PMID: 28149768 Free PMC article.
References
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. - PubMed
-
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

