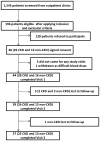
Differences in Whole Blood Platelet Aggregation at Baseline and in Response to Aspirin and Aspirin Plus Clopidogrel in Patients With Versus Without Chronic Kidney Disease
- PMID: 26725101
- PMCID: PMC4738090
- DOI: 10.1016/j.amjcard.2015.11.029
Differences in Whole Blood Platelet Aggregation at Baseline and in Response to Aspirin and Aspirin Plus Clopidogrel in Patients With Versus Without Chronic Kidney Disease
Abstract
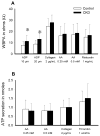
Thrombotic events while receiving antiplatelet agents (APAs) are more common in subjects with versus without chronic kidney disease (CKD). Data on antiplatelet effects of APA in CKD are scarce and limited by lack of baseline platelet function before APA treatment. We hypothesized subjects with stages 4 to 5 CKD versus no CKD have greater baseline platelet aggregability and respond poorly to aspirin and clopidogrel. In a prospective controlled study, we measured whole blood platelet aggregation (WBPA) in 28 CKD and 16 non-CKD asymptomatic stable outpatients not on APA, frequency-matched for age, gender, obesity, and diabetes mellitus. WBPA was remeasured after 2 weeks of each aspirin and aspirin plus clopidogrel. The primary outcome was percent inhibition of platelet aggregation (IPA) from baseline. The secondary outcome was residual platelet aggregability (RPA; proportion with <50% IPA). Baseline platelet aggregability was similar between groups except adenosine diphosphate-induced WBPA, which was higher in CKD versus non-CKD; median (interquartile range) = 13.5 (9.5 to 16.0) versus 9.0 (6.0 to 12.0) Ω, p = 0.007. CKD versus non-CKD participants had lower clopidogrel-induced IPA, 38% versus 72%, p = 0.04. A greater proportion of CKD versus non-CKD participants had RPA after clopidogrel treatment (56% vs 8.3%, p = 0.01). There were no significant interactions between CKD and the presence of cytochrome P450 2C19 polymorphisms for platelet aggregability in clopidogrel-treated participants. In conclusion, CKD versus non-CKD subjects exhibited similar platelet aggregation at baseline, similar aspirin effects and greater RPA on clopidogrel, which was independent of cytochrome P450 2C19 polymorphisms.
Trial registration: ClinicalTrials.gov NCT01768637.
Published by Elsevier Inc.
Figures
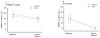
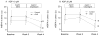
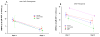
Similar articles
-
Ticagrelor inhibits platelet aggregation and reduces inflammatory burden more than clopidogrel in patients with stages 4 or 5 chronic kidney disease.Vascul Pharmacol. 2023 Feb;148:107143. doi: 10.1016/j.vph.2023.107143. Epub 2023 Jan 20. Vascul Pharmacol. 2023. PMID: 36682595 Free PMC article. Clinical Trial.
-
Platelet inhibition by adjunctive cilostazol versus high maintenance-dose clopidogrel in patients with acute myocardial infarction according to cytochrome P450 2C19 genotype.JACC Cardiovasc Interv. 2011 Apr;4(4):381-91. doi: 10.1016/j.jcin.2010.12.010. JACC Cardiovasc Interv. 2011. PMID: 21511217 Clinical Trial.
-
Preoperative platelet aggregation predicts perioperative blood loss and rethoracotomy for bleeding in patients receiving dual antiplatelet treatment prior to coronary surgery.Thromb Res. 2015 Sep;136(3):519-25. doi: 10.1016/j.thromres.2015.04.037. Epub 2015 May 5. Thromb Res. 2015. PMID: 26003782 Clinical Trial.
-
[high on-treatment platelet reactivity in patients with chronic renal failure using acetylsalicylic acid].Wiad Lek. 2017;70(6 pt 1):1102-1107. Wiad Lek. 2017. PMID: 29478986 Review. Polish.
-
Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence?Clin J Am Soc Nephrol. 2013 Apr;8(4):665-74. doi: 10.2215/CJN.06790712. Epub 2012 Sep 27. Clin J Am Soc Nephrol. 2013. PMID: 23024160 Free PMC article. Review.
Cited by
-
Ticagrelor inhibits platelet aggregation and reduces inflammatory burden more than clopidogrel in patients with stages 4 or 5 chronic kidney disease.Vascul Pharmacol. 2023 Feb;148:107143. doi: 10.1016/j.vph.2023.107143. Epub 2023 Jan 20. Vascul Pharmacol. 2023. PMID: 36682595 Free PMC article. Clinical Trial.
-
Comparative Effectiveness and Safety of Oral P2Y12 Inhibitors in Patients on Chronic Dialysis.Kidney Int Rep. 2021 Jul 3;6(9):2381-2391. doi: 10.1016/j.ekir.2021.06.031. eCollection 2021 Sep. Kidney Int Rep. 2021. PMID: 34514199 Free PMC article.
-
Association of platelet function with depression and its treatment with sertraline in patients with chronic kidney disease: analysis of a randomized trial.BMC Nephrol. 2019 Oct 29;20(1):395. doi: 10.1186/s12882-019-1576-7. BMC Nephrol. 2019. PMID: 31664940 Free PMC article. Clinical Trial.
-
Platelet Activity and Cardiovascular Risk in CKD and Peripheral Artery Disease.Kidney Int Rep. 2022 Aug 4;7(10):2242-2250. doi: 10.1016/j.ekir.2022.07.169. eCollection 2022 Oct. Kidney Int Rep. 2022. PMID: 36217517 Free PMC article.
-
Review on Kidney-Liver Crosstalk: Pathophysiology of Their Disorders.Cell J. 2024 Feb 1;26(2):98-111. doi: 10.22074/cellj.2023.2007757.1376. Cell J. 2024. PMID: 38459727 Free PMC article.
References
-
- Machecourt J, Danchin N, Lablanche JM, Fauvel JM, Bonnet JL, Marliere S, Foote A, Quesada JL, Eltchaninoff H, Vanzetto G, Investigators E. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: the EVASTENT Matched-Cohort Registry. J Am Coll Cardiol. 2002;39:1113–1119. - PubMed
-
- Aradi D, Komocsi A, Vorobcsuk A, Rideg O, Tokes-Fuzesi M, Magyarlaki T, Horvath IG, Serebruany VL. Prognostic significance of high on-clopidogrel platelet reactivity after percutaneous coronary intervention: systematic review and meta-analysis. Am Heart J. 2010;160:543–551. - PubMed
-
- Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez-Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez-Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. - PubMed
-
- Woo JS, Kim W, Lee SR, Jung KH, Kim WS, Lew JH, Lee TW, Lim CK. Platelet reactivity in patients with chronic kidney disease receiving adjunctive cilostazol compared with a high-maintenance dose of clopidogrel: results of the effect of platelet inhibition according to clopidogrel dose in patients with chronic kidney disease (PIANO-2 CKD) randomized study. Am Heart J. 2011;162:1018–1025. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

