Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses
- PMID: 26725514
- PMCID: PMC4698756
- DOI: 10.1038/srep18507
Faecalibacterium prausnitzii A2-165 has a high capacity to induce IL-10 in human and murine dendritic cells and modulates T cell responses
Abstract
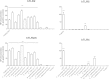
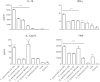
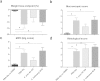
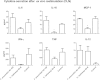
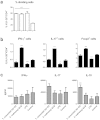
Faecalibacterium prausnitzii strain A2-165 was previously reported to have anti-inflammatory properties and prevent colitis in a TNBS model. We compared the immunomodulatory properties of strain A2-165 to four different F. prausnitzii isolates and eight abundant intestinal commensals using human dendritic cells (DCs) and mouse BMDCs in vitro. Principal component analysis revealed that the cytokine response to F. prausnitzii A2-165 is distinct from the other strains in eliciting high amounts of IL-10 secretion. The mouse DNBS model of relapsing IBD was used to compare the protective effects of F. prausnitzii A2-165 and Clostridium hathewayi, a low secretor of IL-10, on the Th1-driven inflammatory response to DNBS; attenuation of disease parameters was only observed with F. prausnitzii. In an in vivo mouse model of nasal tolerance to ovalbumin, F. prausnitzii A2-165 enhanced ovalbumin-specific T cell proliferation and reduced the proportion of IFN-γ(+) T cells in CLNs. Similarly, in vitro F. prausnitzii A2-165 stimulated BMDCs increased ovalbumin-specific T cell proliferation and reduced the number of IFN-γ(+) T cells. These mechanisms may contribute to the anti-inflammatory effects of F. prausnitzii in colitis and support the notion that this abundant bacterium might contribute to immune homeostasis in the intestine via its anti-inflammatory properties.
Figures



References
-
- Tanaka K. & Ishikawa H. Role of intestinal bacterial flora in oral tolerance induction. Histology and histopathology 19, 907–914 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

