Insights into the channel gating of P2X receptors from structures, dynamics and small molecules
- PMID: 26725734
- PMCID: PMC4722974
- DOI: 10.1038/aps.2015.127
Insights into the channel gating of P2X receptors from structures, dynamics and small molecules
Abstract
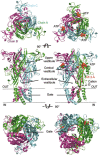
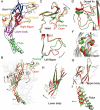
P2X receptors, as ATP-gated non-selective trimeric ion channels, are permeable to Na(+), K(+) and Ca(2+). Comparing with other ligand-gated ion channel families, P2X receptors are distinct in their unique gating properties and pathophysiological roles, and have attracted attention as promising drug targets for a variety of diseases, such as neuropathic pain, multiple sclerosis, rheumatoid arthritis and thrombus. Several small molecule inhibitors for distinct P2X subtypes have entered into clinical trials. However, many questions regarding the gating mechanism of P2X remain unsolved. The structural determinations of P2X receptors at the resting and ATP-bound open states revealed that P2X receptor gating is a cooperative allosteric process involving multiple domains, which marks the beginning of the post-structure era of P2X research at atomic level. Here, we review the current knowledge on the structure-function relationship of P2X receptors, depict the whole picture of allosteric changes during the channel gating, and summarize the active sites that may contribute to new strategies for developing novel allosteric drugs targeting P2X receptors.
Figures


Similar articles
-
Molecular structure and function of P2X receptors.Neuropharmacology. 2016 May;104:18-30. doi: 10.1016/j.neuropharm.2015.07.032. Epub 2015 Jul 29. Neuropharmacology. 2016. PMID: 26231831 Review.
-
P2X Receptor Activation.Adv Exp Med Biol. 2017;1051:55-69. doi: 10.1007/5584_2017_55. Adv Exp Med Biol. 2017. PMID: 28639248 Review.
-
Intersubunit physical couplings fostered by the left flipper domain facilitate channel opening of P2X4 receptors.J Biol Chem. 2017 May 5;292(18):7619-7635. doi: 10.1074/jbc.M116.771121. Epub 2017 Mar 16. J Biol Chem. 2017. PMID: 28302727 Free PMC article.
-
Architectural and functional similarities between trimeric ATP-gated P2X receptors and acid-sensing ion channels.J Mol Biol. 2015 Jan 16;427(1):54-66. doi: 10.1016/j.jmb.2014.06.004. Epub 2014 Jun 14. J Mol Biol. 2015. PMID: 24937752 Review.
-
Geometric rules of channel gating inferred from computational models of the P2X receptor transmembrane domain.J Mol Graph Model. 2015 Sep;61:107-14. doi: 10.1016/j.jmgm.2015.06.015. Epub 2015 Jul 9. J Mol Graph Model. 2015. PMID: 26209765
Cited by
-
How Structural Biology Has Directly Impacted Our Understanding of P2X Receptor Function and Gating.Methods Mol Biol. 2022;2510:1-29. doi: 10.1007/978-1-0716-2384-8_1. Methods Mol Biol. 2022. PMID: 35776317
-
Altered allostery of the left flipper domain underlies the weak ATP response of rat P2X5 receptors.J Biol Chem. 2019 Dec 20;294(51):19589-19603. doi: 10.1074/jbc.RA119.009959. Epub 2019 Nov 14. J Biol Chem. 2019. PMID: 31727741 Free PMC article.
-
A conserved residue in the P2X4 receptor has a nonconserved function in ATP recognition.J Biol Chem. 2021 Jan-Jun;296:100655. doi: 10.1016/j.jbc.2021.100655. Epub 2021 Apr 23. J Biol Chem. 2021. PMID: 33901491 Free PMC article.
-
Alcohol and e-cigarette damage alveolar-epithelial barrier by activation of P2X7r and provoke brain endothelial injury via extracellular vesicles.Res Sq [Preprint]. 2023 Nov 14:rs.3.rs-3552555. doi: 10.21203/rs.3.rs-3552555/v1. Res Sq. 2023. Update in: Cell Commun Signal. 2024 Jan 15;22(1):39. doi: 10.1186/s12964-023-01461-1. PMID: 38014253 Free PMC article. Updated. Preprint.
-
Simulation of P2X-mediated calcium signalling in microglia.J Physiol. 2019 Feb;597(3):799-818. doi: 10.1113/JP277377. Epub 2018 Dec 17. J Physiol. 2019. PMID: 30462840 Free PMC article.
References
-
- 1Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci 2001; 2: 165–74. - PubMed
-
- 2Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006; 442: 527–32. - PubMed
-
- 5Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci 2006; 27: 166–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

