Nonfouling NTA-PEG-Based TEM Grid Coatings for Selective Capture of Histidine-Tagged Protein Targets from Cell Lysates
- PMID: 26726866
- PMCID: PMC5310270
- DOI: 10.1021/acs.langmuir.5b03445
Nonfouling NTA-PEG-Based TEM Grid Coatings for Selective Capture of Histidine-Tagged Protein Targets from Cell Lysates
Abstract
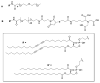
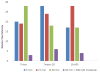
We report the preparation and performance of TEM grids bearing stabilized nonfouling lipid monolayer coatings. These films contain NTA capture ligands of controllable areal density at the distal end of a flexible poly(ethylene glycol) 2000 (PEG2000) spacer to avoid preferred orientation of surface-bound histidine-tagged (His-tag) protein targets. Langmuir-Schaefer deposition at 30 mN/m of mixed monolayers containing two novel synthetic lipids-1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[(5-amido-1-carboxypentyl)iminodiacetic acid]polyethylene glycolamide 2000) (NTA-PEG2000-DSPE) and 1,2-(tricosa-10',12'-diynoyl)-sn-glycero-3-phosphoethanolamine-N-(methoxypolyethylene glycolamide 350) (mPEG350-DTPE)-in 1:99 and 5:95 molar ratios prior to treatment with a 5 min, 254 nm light exposure was used for grid fabrication. These conditions were designed to limit nonspecific protein adsorption onto the stabilized lipid coating by favoring the formation of a mPEG350 brush layer below a flexible, mushroom conformation of NTA-PEG2000 at low surface density to enable specific immobilization and random orientation of the protein target on the EM grid. These grids were then used to capture His6-T7 bacteriophage and RplL from cell lysates, as well as purified His8-green fluorescent protein (GFP) and nanodisc solubilized maltose transporter, His6-MalFGK2. Our findings indicate that TEM grid supported, polymerized NTA lipid monolayers are capable of capturing His-tag protein targets in a manner that controls their areal densities, while efficiently blocking nonspecific adsorption and limiting film degradation, even upon prolonged detergent exposure.
Figures





References
-
- Uzgiris EE, Kornberg RD. Two-dimensional Crystallization Technique for Imaging Macromolecules, With Application to Antigen-Antibody Complement Complexes. Nature. 1983;301:125–129. - PubMed
-
- Thompson DH, Zhou M, Grey J, Kim H-k. Design, Synthesis and Performance of NTA-Modified Lipids as Templates for Histidine-Tagged Protein Crystallization. Chemistry Lett. 2007;36:956–975.
-
- Schmitt L, Dietrich C, Tampe R. Synthesis and Characterization of Chelator-lipids for Reversible Immobilization of Engineered Proteins at Self-assembled Lipid Interfaces. J Am Chem Soc. 1994;116:8485–8491.
-
- Kubalek EW, Le Grice SFJ, Brown PO. Two-dimensional Crystallization of Histidine-tagged, HIV-1 Reverse Transcriptase Promoted by a Novel Nickel-chelating Lipid. J Struct Biol. 1994;113:117–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

