Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity
- PMID: 26726946
- PMCID: PMC4827007
- DOI: 10.1038/bmt.2015.307
Evolving paradigms in the treatment of relapsed/refractory multiple myeloma: increased options and increased complexity
Abstract
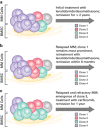
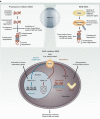
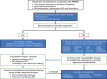
The use of modern therapies such as thalidomide, bortezomib and lenalidomide coupled with upfront high-dose therapy and autologous stem cell transplant (ASCT) has resulted in improved survival in patients with newly diagnosed multiple myeloma (MM). However, patients with relapsed/refractory multiple myeloma (RRMM) often have poorer clinical outcomes and might benefit from novel therapeutic strategies. Emerging therapies, such as deacetylase inhibitors, monoclonal antibodies and new proteasome inhibitors, appear promising and may change the therapeutic landscape in RRMM. A limited number of studies has shown a benefit with salvage ASCT in patients with RRMM, although there remains ongoing debate about its timing and effectiveness. Improvement in transplant outcomes has re-ignited a debate on the timing and possible role for salvage ASCT and allogeneic stem cell transplant in RRMM. As the treatment options for management of patients with RRMM become increasingly complex, physicians must consider both disease- and patient-related factors in choosing the appropriate therapeutic approach, with the goal of improving efficacy while minimizing toxicity.
Conflict of interest statement
RFC declares no potential conflicts of interest. AAK has received research grants from Novartis Pharmaceuticals and Celgene, whose products are discussed in this article.
Figures

References
-
- Katzel JA, Hari P, Vesole DH. Multiple myeloma: charging toward a bright future. CA Cancer J Clin 2007; 57: 301–318. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med 2004; 351: 1860–1873. - PubMed
-
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ et al SEER Cancer Statistics Review, 1975-2004. National Cancer Institute: Bethesda, MD, 2007. Available at http://seer.cancer.gov/csr/1975_2004/.
-
- Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol 2010; 28: 2612–2624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

