Protein Delivery of an Artificial Transcription Factor Restores Widespread Ube3a Expression in an Angelman Syndrome Mouse Brain
- PMID: 26727042
- PMCID: PMC4786922
- DOI: 10.1038/mt.2015.236
Protein Delivery of an Artificial Transcription Factor Restores Widespread Ube3a Expression in an Angelman Syndrome Mouse Brain
Abstract
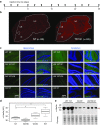
Angelman syndrome (AS) is a neurological genetic disorder caused by loss of expression of the maternal copy of UBE3A in the brain. Due to brain-specific genetic imprinting at this locus, the paternal UBE3A is silenced by a long antisense transcript. Inhibition of the antisense transcript could lead to unsilencing of paternal UBE3A, thus providing a therapeutic approach for AS. However, widespread delivery of gene regulators to the brain remains challenging. Here, we report an engineered zinc finger-based artificial transcription factor (ATF) that, when injected i.p. or s.c., crossed the blood-brain barrier and increased Ube3a expression in the brain of an adult mouse model of AS. The factor displayed widespread distribution throughout the brain. Immunohistochemistry of both the hippocampus and cerebellum revealed an increase in Ube3a upon treatment. An ATF containing an alternative DNA-binding domain did not activate Ube3a. We believe this to be the first report of an injectable engineered zinc finger protein that can cause widespread activation of an endogenous gene in the brain. These observations have important implications for the study and treatment of AS and other neurological disorders.
Figures



References
-
- Angelman, H (1965). ‘Puppet' children. A report on three cases. Dev Med Child Neurol 7: 681–688. - PubMed
-
- Kishino, T, Lalande, M and Wagstaff, J (1997). UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet 15: 70–73. - PubMed
-
- Albrecht, U, Sutcliffe, JS, Cattanach, BM, Beechey, CV, Armstrong, D, Eichele, G et al. (1997). Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat Genet 17: 75–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

