Helicobacter pylori and CagA under conditions of iron deficiency
- PMID: 26727420
- PMCID: PMC4826123
- DOI: 10.1080/19490976.2015.1105426
Helicobacter pylori and CagA under conditions of iron deficiency
Erratum in
- Addendum to: Noto JM, Lee JY, Gaddy JA, Cover TL, Amieva MR, Peek RM Jr. Regulation of Helicobacter pylori Virulence Within the Context of Iron Deficiency. J Infect Dis 2015; 211(11):1790-4
Abstract
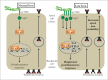
Iron deficiency is the most common nutritional deficiency worldwide and compelling evidence has demonstrated that this condition heightens the risk of gastric cancer. Infection with Helicobacter pylori is the strongest known risk factor for the development of gastric adenocarcinoma. Recent work has demonstrated that, under conditions of iron deficiency, H. pylori-induced gastric carcinogenesis is augmented through increased formation of the strain-specific cag type IV secretion system and enhanced delivery of the bacterial oncoprotein CagA into host cells. Although CagA is a potent virulence factor that promotes oncogenic responses, additional studies have now demonstrated that CagA modulates host cell iron homeostasis in vitro and fundamental metabolic functions of the bacterial cell in vivo. Here we discuss these findings and describe working models by which CagA exerts its effects on gastric epithelial cells, with particular emphasis on its potential role in modulation of host iron homeostasis.
Keywords: CagA; Helicobacter pylori; cag type IV secretion system, gastric cancer; iron deficiency.
Figures


Similar articles
-
Modification of the Gastric Mucosal Microbiota by a Strain-Specific Helicobacter pylori Oncoprotein and Carcinogenic Histologic Phenotype.mBio. 2019 May 28;10(3):e00955-19. doi: 10.1128/mBio.00955-19. mBio. 2019. PMID: 31138752 Free PMC article.
-
Role of Helicobacter pylori virulence factors for iron acquisition from gastric epithelial cells of the host and impact on bacterial colonization.Future Microbiol. 2011 Aug;6(8):843-6. doi: 10.2217/fmb.11.75. Future Microbiol. 2011. PMID: 21861616
-
Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis.Cell Host Microbe. 2014 Mar 12;15(3):306-16. doi: 10.1016/j.chom.2014.02.008. Cell Host Microbe. 2014. PMID: 24629337 Review.
-
Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways.Cell Commun Signal. 2015 Jul 11;13:30. doi: 10.1186/s12964-015-0111-0. Cell Commun Signal. 2015. PMID: 26160167 Free PMC article. Review.
-
Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway.J Exp Clin Cancer Res. 2018 Nov 22;37(1):280. doi: 10.1186/s13046-018-0962-5. J Exp Clin Cancer Res. 2018. PMID: 30466467 Free PMC article.
Cited by
-
IRON DEFICIENCY PROMOTES HELICOBACTER PYLORI-INDUCED GASTRIC CARCINOGENESIS BY ENABLING RECIPROCITY BETWEEN CARCINOGENIC SECONDARY BILE ACIDS AND PROTECTIVE LONG-CHAIN FATTY ACIDS.Trans Am Clin Climatol Assoc. 2025;135:206-221. Trans Am Clin Climatol Assoc. 2025. PMID: 40771613 Free PMC article.
-
Infection of Helicobacter pylori contributes to the progression of gastric cancer through ferroptosis.Cell Death Discov. 2024 Dec 2;10(1):485. doi: 10.1038/s41420-024-02253-3. Cell Death Discov. 2024. PMID: 39622791 Free PMC article. Review.
-
Clinical Pathogenesis, Molecular Mechanisms of Gastric Cancer Development.Curr Top Microbiol Immunol. 2023;444:25-52. doi: 10.1007/978-3-031-47331-9_2. Curr Top Microbiol Immunol. 2023. PMID: 38231214 Free PMC article.
References
-
- Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet 2015; PMID: 26314490; http://dx.doi.org/doi: 2522084210.1016/S0140-6736(15)60865-0 - DOI - PubMed
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359-86; PMID:25220842; http://dx.doi.org/10.1002/ijc.29210 - DOI - PubMed
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87-108; PMID:25651787; http://dx.doi.org/10.3322/caac.21262 - DOI - PubMed
-
- Fernandez-Real JM, Manco M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol 2014; 2:513-26; PMID:24731656; http://dx.doi.org/10.1016/S2213-8587(13)70174-8 - DOI - PubMed
-
- von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015; 12(11):659-69; PMID: 26194551; http://dx.doi.org/doi: 200156210.1038/nrcardio.2015.109 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
