The Impact of Prenatal Ethanol Exposure on Neuroanatomical and Behavioral Development in Mice
- PMID: 26727530
- PMCID: PMC4702517
- DOI: 10.1111/acer.12936
The Impact of Prenatal Ethanol Exposure on Neuroanatomical and Behavioral Development in Mice
Abstract
Background: In utero alcohol, or ethanol (EtOH), exposure produces developmental abnormalities in the brain of the fetus, which can result in lifelong behavioral abnormalities. Fetal alcohol spectrum disorders (FASD) is a term used to describe a range of adverse developmental conditions caused by EtOH exposure during gestation. Children diagnosed with FASD potentially exhibit a host of phenotypes including growth retardation, facial dysmorphology, central nervous system anomalies, abnormal behavior, and cognitive deficits. Previous research suggests that abnormal gene expression and circuitry in the neocortex may underlie reported disabilities of learning, memory, and behavior resulting from early exposure to alcohol (J Neurosci, 33, 2013, 18893).
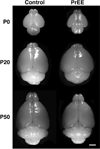
Methods: Here, we utilize a mouse model of FASD to examine effects of prenatal EtOH exposure (PrEE), on brain anatomy in newborn (postnatal day [P]0), weanling (P20), and early adult (P50) mice. We correlate abnormal cortical and subcortical anatomy with atypical behavior in adult P50 PrEE mice. In this model, experimental dams self-administered a 25% EtOH solution throughout gestation (gestational days 0 to 19, day of birth), generating the exposure to the offspring.
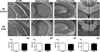
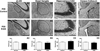
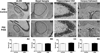
Results: Results from these experiments reveal long-term alterations to cortical anatomy, including atypical developmental cortical thinning, and abnormal subcortical development as a result of in utero EtOH exposure. Furthermore, offspring exposed to EtOH during the prenatal period performed poorly on behavioral tasks measuring sensorimotor integration and anxiety.
Conclusions: Insight from this study will help provide new information on developmental trajectories of PrEE and the biological etiologies of abnormal behavior in people diagnosed with FASD.
Keywords: Anatomy; Brain Development; Fetal Alcohol Spectrum Disorders; Neocortex; Prenatal Alcohol Exposure.
Copyright © 2016 by the Research Society on Alcoholism.
Figures






Comment in
-
Commentary: Linking Cortical and Subcortical Developmental Trajectories to Behavioral Deficits in a Mouse Model of Prenatal Alcohol Exposure.Alcohol Clin Exp Res. 2016 Mar;40(3):448-50. doi: 10.1111/acer.12993. Epub 2016 Feb 5. Alcohol Clin Exp Res. 2016. PMID: 26849677 Free PMC article. No abstract available.
References
-
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43:148–154. - PubMed
-
- Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: A morphological study. Brain Res. 1977;119(2):249–268. - PubMed
-
- Bookstein FL, Connor PD, Huggins JE, Barr HM, Pimentel KD, Streissguth AP. Many infants prenatally exposed to high levels of alcohol show one particular anomaly of the corpus callosum. Alcohol Clin Exp Res. 2007;31:868–879. - PubMed
-
- Bookstein FL, Sampson PD, Connor PD, Streissguth AP. Midline corpus callosum is a neuroanatomical focus of fetal alcohol damage. Anat Rec Part B New Anat. 2002;269:162–174. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

