Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics
- PMID: 26727948
- PMCID: PMC4700584
- DOI: 10.1186/s40478-015-0269-0
Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics
Erratum in
-
Erratum: Spatial genomic heterogeneity in diffuse intrinsic pontine and midline high-grade glioma: implications for diagnostic biopsy and targeted therapeutics.Acta Neuropathol Commun. 2016 Feb 9;4:13. doi: 10.1186/s40478-016-0283-x. Acta Neuropathol Commun. 2016. PMID: 26860432 Free PMC article.
Abstract
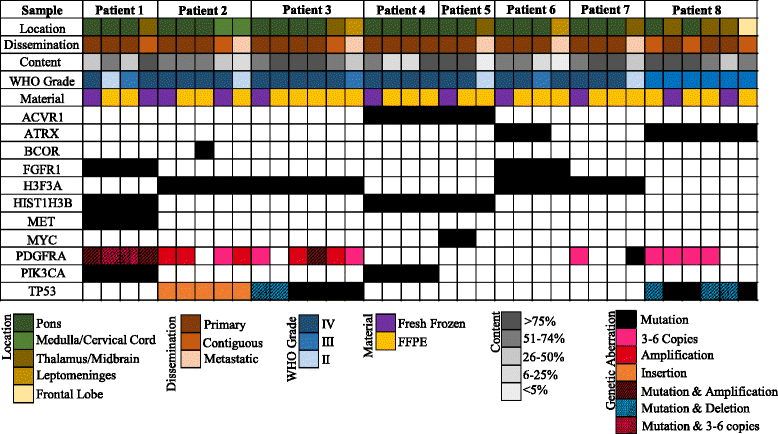
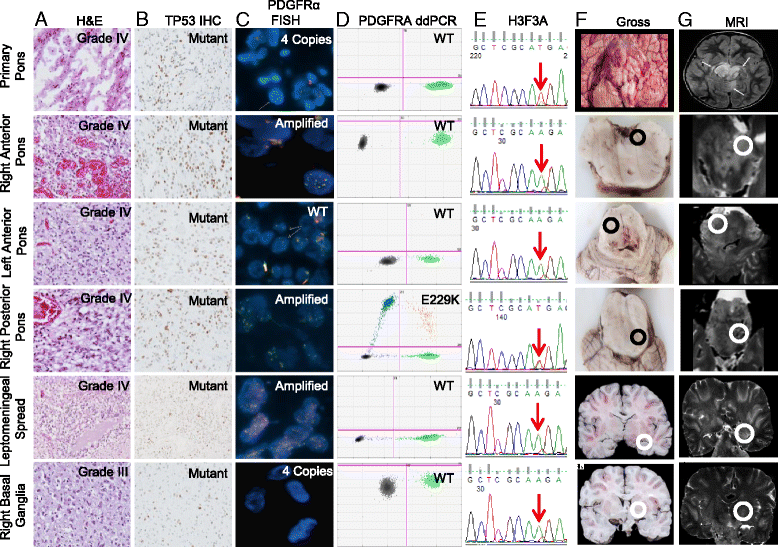
Introduction: Diffuse intrinsic pontine glioma (DIPG) and midline high-grade glioma (mHGG) are lethal childhood brain tumors. Spatial genomic heterogeneity has been well-described in adult HGG but has not been comprehensively characterized in pediatric HGG. We performed whole exome sequencing on 38-matched primary, contiguous, and metastatic tumor sites from eight children with DIPG (n = 7) or mHGG (n = 1) collected using a unique MRI-guided autopsy protocol. Validation was performed using Sanger sequencing, Droplet Digital polymerase-chain reaction, immunohistochemistry, and fluorescent in-situ hybridization.
Results: Median age at diagnosis was 6.1 years (range: 2.9-23.3 years). Median overall survival was 13.2 months (range: 11.2-32.2 months). Contiguous tumor infiltration and distant metastases were observed in seven and six patients, respectively, including leptomeningeal dissemination in three DIPGs. Histopathological heterogeneity was evident in seven patients, including intra-pontine heterogeneity in two DIPGs, ranging from World Health Organization grade II to IV astrocytoma. We found conservation of heterozygous K27M mutations in H3F3A (n = 4) or HIST1H3B (n = 3) across all primary, contiguous, and metastatic tumor sites in all DIPGs. ACVR1 (n = 2), PIK3CA (n = 2), FGFR1 (n = 2), and MET (n = 1) were also intra-tumorally conserved. ACVR1 was co-mutated with HIST1H3B (n = 2). In contrast, PDGFRA amplification and mutation were spatially heterogeneous, as were mutations in BCOR (n = 1), ATRX (n = 2), and MYC (n = 1). TP53 aberrations (n = 3 patients) varied by type and location between primary and metastatic tumors sites but were intra-tumorally conserved.
Conclusion: Spatial conservation of prognostically-relevant and therapeutically-targetable somatic mutations in DIPG and mHGG contrasts the significant heterogeneity of driver mutations seen in adult HGG and supports uniform implementation of diagnostic biopsy in DIPG and mHGG to classify molecular risk groups and guide therapeutic strategy.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

