Wilson-Cowan Equations for Neocortical Dynamics
- PMID: 26728012
- PMCID: PMC4733815
- DOI: 10.1186/s13408-015-0034-5
Wilson-Cowan Equations for Neocortical Dynamics
Abstract
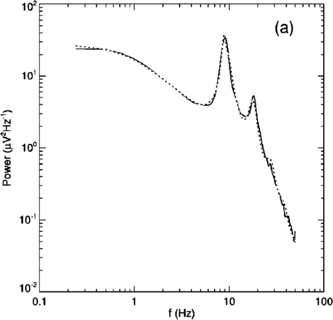
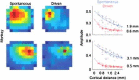
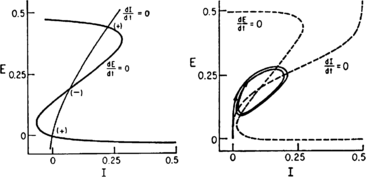
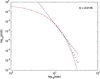
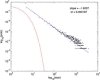
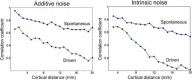
In 1972-1973 Wilson and Cowan introduced a mathematical model of the population dynamics of synaptically coupled excitatory and inhibitory neurons in the neocortex. The model dealt only with the mean numbers of activated and quiescent excitatory and inhibitory neurons, and said nothing about fluctuations and correlations of such activity. However, in 1997 Ohira and Cowan, and then in 2007-2009 Buice and Cowan introduced Markov models of such activity that included fluctuation and correlation effects. Here we show how both models can be used to provide a quantitative account of the population dynamics of neocortical activity.We first describe how the Markov models account for many recent measurements of the resting or spontaneous activity of the neocortex. In particular we show that the power spectrum of large-scale neocortical activity has a Brownian motion baseline, and that the statistical structure of the random bursts of spiking activity found near the resting state indicates that such a state can be represented as a percolation process on a random graph, called directed percolation.Other data indicate that resting cortex exhibits pair correlations between neighboring populations of cells, the amplitudes of which decay slowly with distance, whereas stimulated cortex exhibits pair correlations which decay rapidly with distance. Here we show how the Markov model can account for the behavior of the pair correlations.Finally we show how the 1972-1973 Wilson-Cowan equations can account for recent data which indicates that there are at least two distinct modes of cortical responses to stimuli. In mode 1 a low intensity stimulus triggers a wave that propagates at a velocity of about 0.3 m/s, with an amplitude that decays exponentially. In mode 2 a high intensity stimulus triggers a larger response that remains local and does not propagate to neighboring regions.
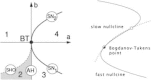
Keywords: Bogdanov–Takens bifurcation; Directed percolation phase transition; Localized decaying LFP and VSD responses; Neural network master equation; Pair-correlations; Propagating decaying LFP and VSD waves; Wilson–Cowan equations.
Figures




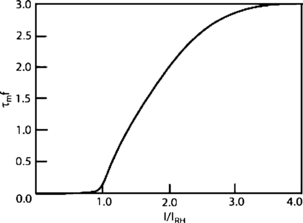


References
-
- Sholl DA. The organization of the cerebral cortex. London: Methuen; 1956.
-
- Caton R. Br Med J. 1875;2:278.
-
- Berger H. Arch Psychiatr Nervenkrankh. 1929;87:527–570. doi: 10.1007/BF01797193. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

