Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model
- PMID: 26728181
- PMCID: PMC4700622
- DOI: 10.1186/s12974-015-0467-5
Non-alcoholic fatty liver disease induces signs of Alzheimer's disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disease afflicting about one third of the world's population and 30 % of the US population. It is induced by consumption of high-lipid diets and is characterized by liver inflammation and subsequent liver pathology. Obesity and consumption of a high-fat diet are known to increase the risk of Alzheimer's disease (AD). Here, we investigated NAFLD-induced liver inflammation in the pathogenesis of AD.
Methods: WT and APP-Tg mice were fed with a standard diet (SD) or a high-fat diet (HFD) for 2, 5 months, or 1 year to induce NAFLD. Another set of APP-Tg mice were removed from HFD after 2 months and put back on SD for 3 months.
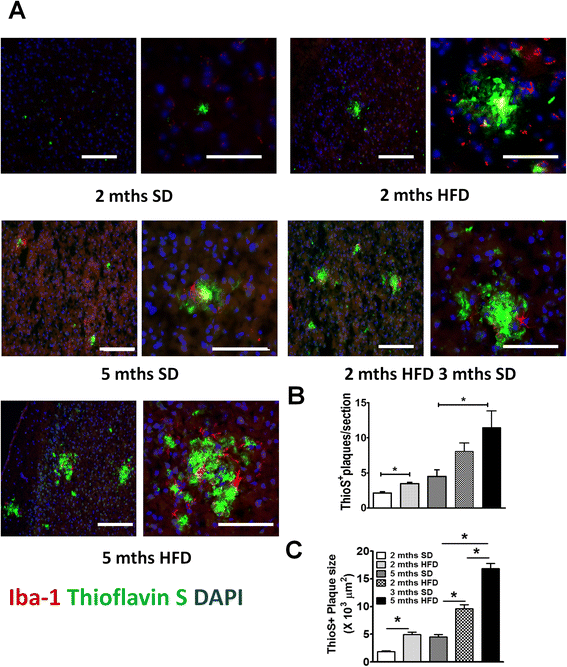
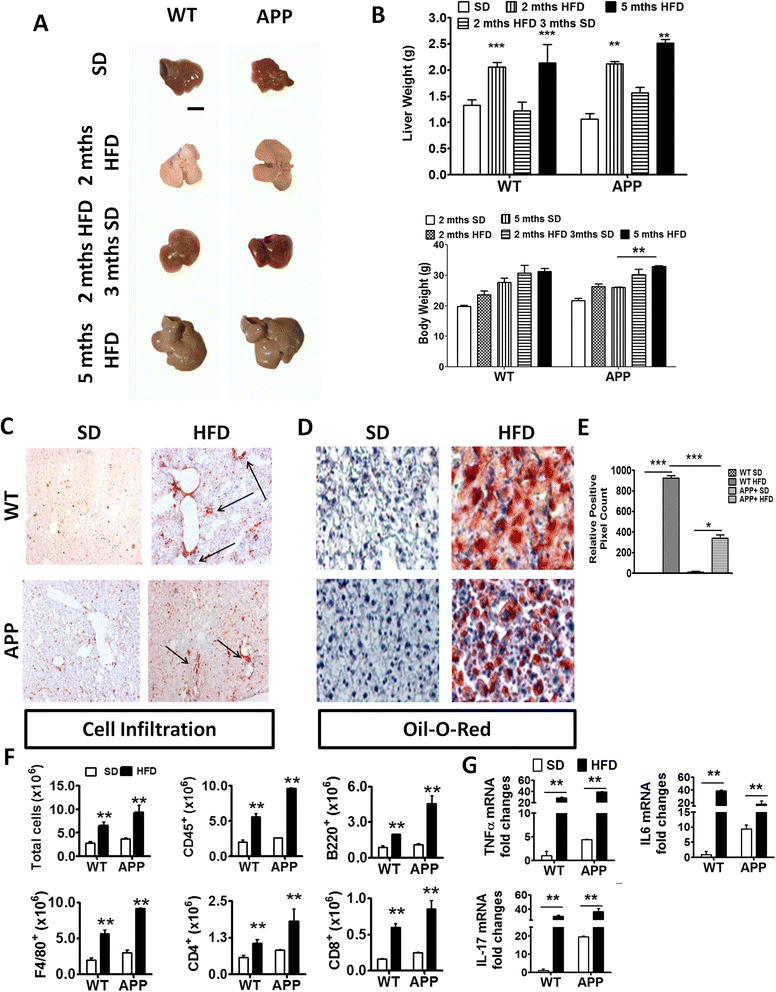
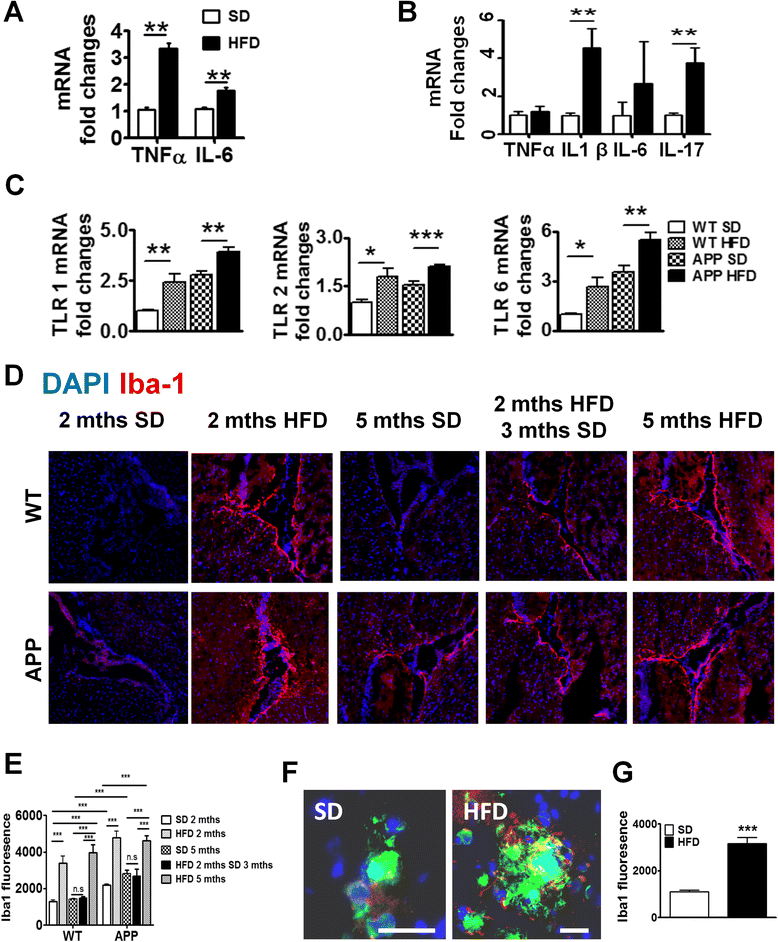
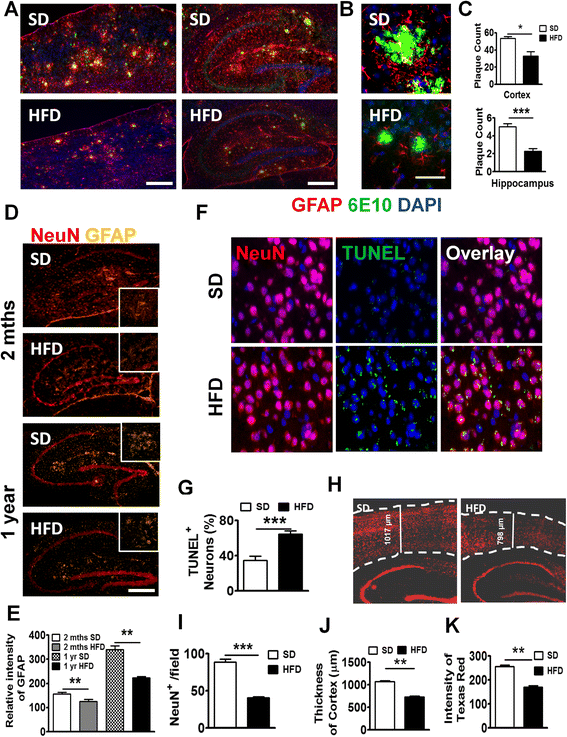
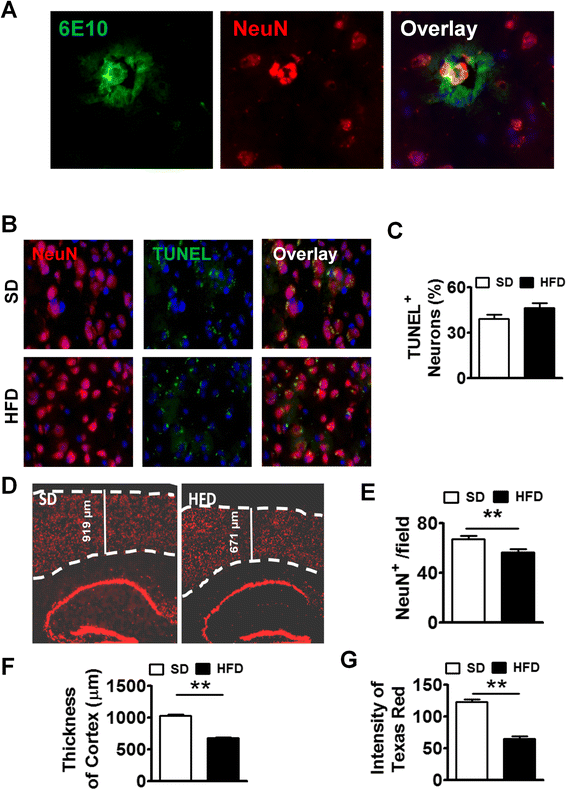
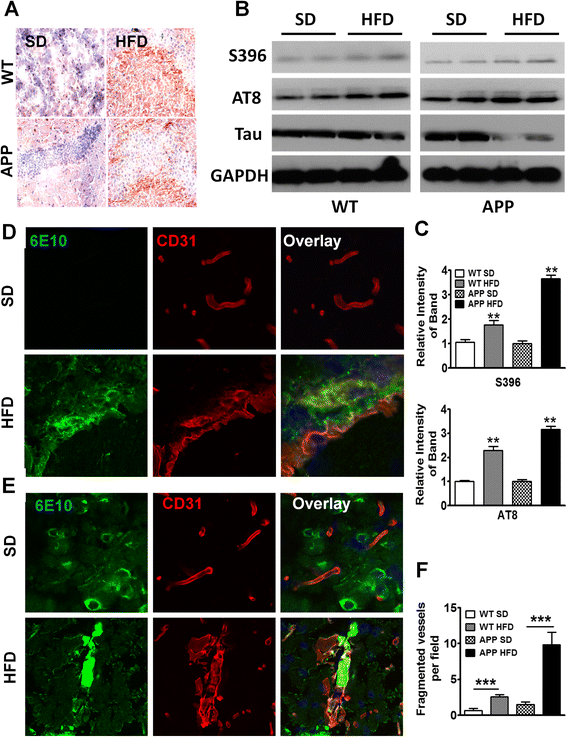
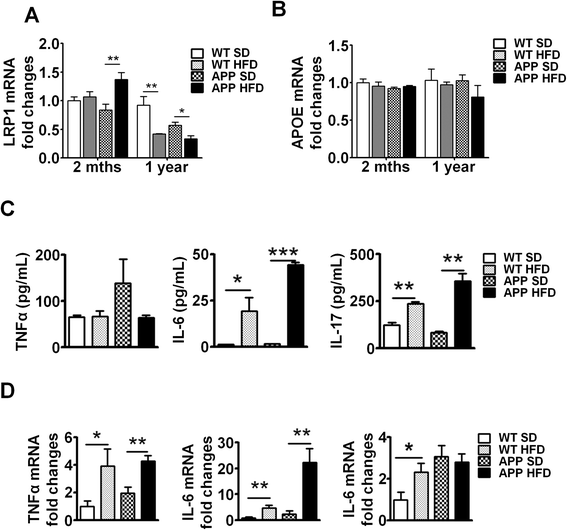
Results: During acute phase NAFLD, WT and APP-Tg mice developed significant liver inflammation and pathology that coincided with increased numbers of activated microglial cells in the brain, increased inflammatory cytokine profile, and increased expression of toll-like receptors. Chronic NAFLD induced advanced pathological signs of AD in both WT and APP-Tg mice, and also induced neuronal apoptosis. We observed decreased brain expression of low-density lipoprotein receptor-related protein-1 (LRP-1) which is involved in β-amyloid clearance, in both WT and APP-Tg mice after ongoing administration of the HFD. LRP-1 expression correlated with advanced signs of AD over the course of chronic NAFLD. Removal of mice from HFD during acute NAFLD reversed liver pathology, decreased signs of activated microglial cells and neuro-inflammation, and decreased β-amyloid plaque load.
Conclusions: Our findings indicate that chronic inflammation induced outside the brain is sufficient to induce neurodegeneration in the absence of genetic predisposition.
Figures
Similar articles
-
The effect of focal brain injury on beta-amyloid plaque deposition, inflammation and synapses in the APP/PS1 mouse model of Alzheimer's disease.Exp Neurol. 2015 May;267:219-29. doi: 10.1016/j.expneurol.2015.02.034. Epub 2015 Mar 4. Exp Neurol. 2015. PMID: 25747037
-
Human Alzheimer's disease gene expression signatures and immune profile in APP mouse models: a discrete transcriptomic view of Aβ plaque pathology.J Neuroinflammation. 2018 Sep 6;15(1):256. doi: 10.1186/s12974-018-1265-7. J Neuroinflammation. 2018. PMID: 30189875 Free PMC article.
-
Chronic Sleep Deprivation Exacerbates Learning-Memory Disability and Alzheimer's Disease-Like Pathologies in AβPP(swe)/PS1(ΔE9) Mice.J Alzheimers Dis. 2016;50(3):669-85. doi: 10.3233/JAD-150774. J Alzheimers Dis. 2016. PMID: 26757041
-
Microglial toll-like receptors and Alzheimer's disease.Brain Behav Immun. 2016 Feb;52:187-198. doi: 10.1016/j.bbi.2015.10.010. Epub 2015 Oct 23. Brain Behav Immun. 2016. PMID: 26526648 Review.
-
Genetically altered transgenic models of Alzheimer's disease.J Neural Transm Suppl. 2000;59:175-83. doi: 10.1007/978-3-7091-6781-6_20. J Neural Transm Suppl. 2000. PMID: 10961430 Review.
Cited by
-
Bidirectional two-sample mendelian randomization analysis identifies causal associations of MRI-based cortical thickness and surface area relation to NAFLD.Lipids Health Dis. 2024 Feb 23;23(1):58. doi: 10.1186/s12944-024-02043-x. Lipids Health Dis. 2024. PMID: 38395962 Free PMC article.
-
Deciphering the Link Between Hyperhomocysteinemia and Ceramide Metabolism in Alzheimer-Type Neurodegeneration.Front Neurol. 2019 Jul 31;10:807. doi: 10.3389/fneur.2019.00807. eCollection 2019. Front Neurol. 2019. PMID: 31417486 Free PMC article.
-
On entropy and information in gene interaction networks.Bioinformatics. 2019 Mar 1;35(5):815-822. doi: 10.1093/bioinformatics/bty691. Bioinformatics. 2019. PMID: 30102349 Free PMC article.
-
Imaging Methods Applicable in the Diagnostics of Alzheimer's Disease, Considering the Involvement of Insulin Resistance.Int J Mol Sci. 2023 Feb 7;24(4):3325. doi: 10.3390/ijms24043325. Int J Mol Sci. 2023. PMID: 36834741 Free PMC article. Review.
-
Impact of diabetes mellitus and chronic liver disease on the incidence of dementia and all-cause mortality among patients with dementia.Medicine (Baltimore). 2017 Nov;96(47):e8753. doi: 10.1097/MD.0000000000008753. Medicine (Baltimore). 2017. PMID: 29381970 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

