Nicotinic Acid Adenine Dinucleotide Phosphate Plays a Critical Role in Naive and Effector Murine T Cells but Not Natural Regulatory T Cells
- PMID: 26728458
- PMCID: PMC4813477
- DOI: 10.1074/jbc.M115.681833
Nicotinic Acid Adenine Dinucleotide Phosphate Plays a Critical Role in Naive and Effector Murine T Cells but Not Natural Regulatory T Cells
Abstract
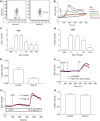
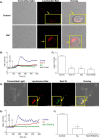
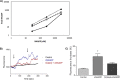
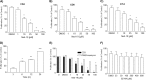
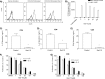
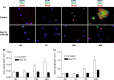
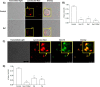
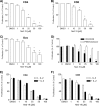
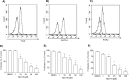
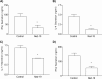
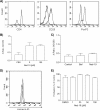
Nicotinic acid adenine dinucleotide phosphate (NAADP), the most potent Ca(2+) mobilizing second messenger discovered to date, has been implicated in Ca(2+) signaling in some lymphomas and T cell clones. In contrast, the role of NAADP in Ca(2+) signaling or the identity of the Ca(2+) stores targeted by NAADP in conventional naive T cells is less clear. In the current study, we demonstrate the importance of NAADP in the generation of Ca(2+) signals in murine naive T cells. Combining live-cell imaging methods and a pharmacological approach using the NAADP antagonist Ned-19, we addressed the involvement of NAADP in the generation of Ca(2+) signals evoked by TCR stimulation and the role of this signal in downstream physiological end points such as proliferation, cytokine production, and other responses to stimulation. We demonstrated that acidic compartments in addition to the endoplasmic reticulum were the Ca(2+) stores that were sensitive to NAADP in naive T cells. NAADP was shown to evoke functionally relevant Ca(2+) signals in both naive CD4 and naive CD8 T cells. Furthermore, we examined the role of this signal in the activation, proliferation, and secretion of effector cytokines by Th1, Th2, Th17, and CD8 effector T cells. Overall, NAADP exhibited a similar profile in mediating Ca(2+) release in effector T cells as in their counterpart naive T cells and seemed to be equally important for the function of these different subsets of effector T cells. This profile was not observed for natural T regulatory cells.
Keywords: Ned-19; T cell; calcium imaging; calcium intracellular release; cellular immune response; nicotinic acid adenine dinucleotide phosphate (NAADP).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Feske S. (2007) Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 7, 690–702 - PubMed
-
- Robert V., Triffaux E., Savignac M., and Pelletier L. (2011) Calcium signalling in T-lymphocytes. Biochimie 93, 2087–2094 - PubMed
-
- Gasser A., Bruhn S., and Guse A. H. (2006) Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 281, 16906–16913 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

