Moving Beyond the Androgen Receptor (AR): Targeting AR-Interacting Proteins to Treat Prostate Cancer
- PMID: 26728473
- PMCID: PMC5380740
- DOI: 10.1007/s12672-015-0239-9
Moving Beyond the Androgen Receptor (AR): Targeting AR-Interacting Proteins to Treat Prostate Cancer
Abstract
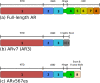
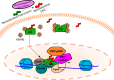
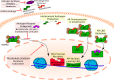
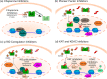
Medical or surgical castration serves as the backbone of systemic therapy for advanced and metastatic prostate cancer, taking advantage of the importance of androgen signaling in this disease. Unfortunately, resistance to castration emerges almost universally. Despite the development and approval of new and more potent androgen synthesis inhibitors and androgen receptor (AR) antagonists, prostate cancers continue to develop resistance to these therapeutics, while often maintaining their dependence on the AR signaling axis. This highlights the need for innovative therapeutic approaches that aim to continue disrupting AR downstream signaling but are orthogonal to directly targeting the AR itself. In this review, we discuss the preclinical research that has been done, as well as clinical trials for prostate cancer, on inhibiting several important families of AR-interacting proteins, including chaperones (such as heat shock protein 90 (HSP90) and FKBP52), pioneer factors (including forkhead box protein A1 (FOXA1) and GATA-2), and AR transcriptional coregulators such as the p160 steroid receptor coactivators (SRCs) SRC-1, SRC-2, SRC-3, as well as lysine deacetylases (KDACs) and lysine acetyltransferases (KATs). Researching the effect of-and developing new therapeutic agents that target-the AR signaling axis is critical to advancing our understanding of prostate cancer biology, to continue to improve treatments for prostate cancer and for overcoming castration resistance.
Conflict of interest statement
All authors state that they have no relevant financial interests to disclose.
Figures
References
-
- Society AC. Cancer facts & figures. Am Cancer Soc. 2014;2014:1–72.
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015 - Siegel - 2015 - CA: a Cancer Journal for Clinicians - Wiley Online Library. Cancer J Clin. 2015 - PubMed
-
- Huggins C, Hodges CV (1941) Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941:9–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

