Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria
- PMID: 26728712
- PMCID: PMC4700437
- DOI: 10.1038/srep18877
Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria
Abstract
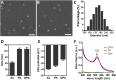
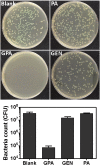
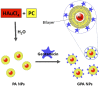
The chronic infections related to biofilm and intracellular bacteria are always hard to be cured because of their inherent resistance to both antimicrobial agents and host defenses. Herein we develop a facile approach to overcome the above conundrum through phosphatidylcholine-decorated Au nanoparticles loaded with gentamicin (GPA NPs). The nanoparticles were characterized by scanning electron microscopy (SEM), dynamic light scattering (DLS) and ultraviolet-visible (UV-vis) absorption spectra which demonstrated that GPA NPs with a diameter of approximately 180 nm were uniform. The loading manner and release behaviors were also investigated. The generated GPA NPs maintained their antibiotic activities against planktonic bacteria, but more effective to damage established biofilms and inhibited biofilm formation of pathogens including Gram-positive and Gram-negative bacteria. In addition, GPA NPs were observed to be nontoxic to RAW 264.7 cells and readily engulfed by the macrophages, which facilitated the killing of intracellular bacteria in infected macrophages. These results suggested GPA NPs might be a promising antibacterial agent for effective treatment of chronic infections due to microbial biofilm and intracellular bacteria.
Figures





Similar articles
-
Facile green synthesis of baicalein fabricated gold nanoparticles and their antibiofilm activity against Pseudomonas aeruginosa PAO1.Microb Pathog. 2017 Jun;107:261-269. doi: 10.1016/j.micpath.2017.03.044. Epub 2017 Apr 2. Microb Pathog. 2017. PMID: 28377235
-
Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria.Int J Biol Macromol. 2020 Aug 1;156:640-647. doi: 10.1016/j.ijbiomac.2020.04.090. Epub 2020 Apr 15. Int J Biol Macromol. 2020. PMID: 32304789
-
Improvement of anti-biofilm activities via co-delivery of curcumin and gentamicin in lipid-polymer hybrid nanoparticle.J Biomater Sci Polym Ed. 2022 Feb;33(2):174-196. doi: 10.1080/09205063.2021.1982159. Epub 2021 Oct 3. J Biomater Sci Polym Ed. 2022. PMID: 34605363
-
Nanomaterials for alternative antibacterial therapy.Int J Nanomedicine. 2017 Nov 10;12:8211-8225. doi: 10.2147/IJN.S132163. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29184409 Free PMC article. Review.
-
A mechanistic perspective on targeting bacterial drug resistance with nanoparticles.J Drug Target. 2021 Nov;29(9):941-959. doi: 10.1080/1061186X.2021.1895818. Epub 2021 Mar 11. J Drug Target. 2021. PMID: 33703979 Review.
Cited by
-
Inorganic nanohybrids combat antibiotic-resistant bacteria hiding within human macrophages.Nanoscale. 2021 May 7;13(17):8224-8234. doi: 10.1039/d0nr08285f. Epub 2021 Apr 22. Nanoscale. 2021. PMID: 33885075 Free PMC article.
-
Antibacterial Pathways in Transition Metal-Based Nanocomposites: A Mechanistic Overview.Int J Nanomedicine. 2022 Dec 30;17:6821-6842. doi: 10.2147/IJN.S392081. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36605560 Free PMC article. Review.
-
Innovative Insights into In Vitro Activity of Colloidal Platinum Nanoparticles against ESBL-Producing Strains of Escherichia coli and Klebsiella pneumoniae.Pharmaceutics. 2022 Aug 17;14(8):1714. doi: 10.3390/pharmaceutics14081714. Pharmaceutics. 2022. PMID: 36015339 Free PMC article.
-
Specific Anti-biofilm Activity of Carbon Quantum Dots by Destroying P. gingivalis Biofilm Related Genes.Int J Nanomedicine. 2020 Jul 31;15:5473-5489. doi: 10.2147/IJN.S253416. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32801701 Free PMC article.
-
Antibacterial evaluation of silver nanoparticles synthesized from lychee peel: individual versus antibiotic conjugated effects.World J Microbiol Biotechnol. 2018 Jul 14;34(8):118. doi: 10.1007/s11274-018-2500-1. World J Microbiol Biotechnol. 2018. PMID: 30008019
References
-
- Lasa I. Towards the identification of the common features of bacterial biofilm development. International Microbiology 9, 21–28 (2006). - PubMed
-
- Mittelman M. W. Structure and Functional Characteristics of Bacterial Biofilms in Fluid Processing Operations. Journal of dairy science 81, 2760–2764 (1998). - PubMed
-
- Van Houdt R. & Michiels C. W. Biofilm formation and the food industry, a focus on the bacterial outer surface. Journal of applied microbiology 109, 1117–1131 (2010). - PubMed
-
- Flemming H.-C. & Wingender J. The biofilm matrix. Nature Reviews Microbiology 8, 623–633 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

