Switching Between Epoetins: A Practice in Support of Biosimilar Use
- PMID: 26728875
- PMCID: PMC4746226
- DOI: 10.1007/s40259-015-0155-0
Switching Between Epoetins: A Practice in Support of Biosimilar Use
Abstract
Background: The acceptability of switching between reference drugs and their biosimilars is often disputed. It is unclear whether this concern is specific to the use of biosimilars or is relevant to the practice of switching between any biological drugs.
Objective: The objective of this study was to quantify the occurrence of switching between different erythropoiesis-stimulating agents.
Methods: A retrospective drug utilization study was conducted in the Umbria region (Italy). The study population included all residents who received their first epoetin prescription between 1 July 2011 and 31 December 2014. The Umbria drug prescription database and the regional archive of residents were used to gather information. Switching was defined as any transition between different epoetins (different substances and/or different products of the same substance) in a series of two prescriptions. The probability of switching was described in relationship to the duration of treatment in a survival analysis.
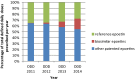
Results: Overall, 3258 subjects received prescriptions of epoetins. Among the 2896 patients with at least two prescriptions, 354 (12.2%) experienced one or more switches. The probability of switching depended on the duration of treatment: approximately 15% of users switched within 12 months of observation and 25% switched within 2 years. Switching was not limited to reference and biosimilar epoetins and it affected patent and off-patent epoetins equally.
Conclusions: Switching between different epoetins was related to the duration of use and most episodes of switching involved epoetins that have never been contrasted in a comparability exercise. The present level of switching may provide reassurance to physicians when taken together with other sources of comparative evidence.
Figures

References
-
- European Medicines Agency. Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant erythropoietins. (EMEA/CHMP/BMWP/301636/2008). 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... Accessed 5 Aug 2015.
-
- AIFA position paper. I farmaci biosmilari. 2013. http://www.agenziafarmaco.gov.it/sites/default/files/AIFA_POSITION_PAPER.... Accessed 5 Aug 2015.
-
- Farmaci biosimilari: sicurezza, efficacia, omogeneità di trattamento e corretta informazione. Un Manifesto dei diritti dei pazienti. 2014. http://www.amrer.it/download_documenti/manifesto-farmaci-biosimilari.pdf. Accessed 17 Dec 2015.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

