A deleterious role for Th9/IL-9 in hepatic fibrogenesis
- PMID: 26728971
- PMCID: PMC4700496
- DOI: 10.1038/srep18694
A deleterious role for Th9/IL-9 in hepatic fibrogenesis
Abstract
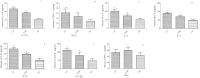
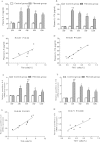
T helper 9 (Th9) cells, a recently recognized Th cell subset, are involved in autoimmune diseases. We aimed to investigate the role of Th9/interleukin-9 (IL-9) in the pathogenesis of hepatic fibrosis. Th9 and Th17 cells were quantified in chronic hepatitis B (CHB) patients with hepatic fibrosis, HBV-associated liver cirrhosis (LC) patients and healthy controls (HC). The percentages of Th9 and Th17 cells, concentrations of IL-9 and IL-17, as well as expression of IL-17, TNF-α, IL-6, IL-4, IL-21, TGF-β1 and IFN-γ were significantly increased in plasma of CHB and LC patients compared with those in HC. Splenic Th9 and Th17 cells, plasma concentrations and liver expression of IL-9 and IL-17A were significantly elevated in mice with hepatic fibrosis compared with controls. Neutralization of IL-9 in mice ameliorated hepatic fibrosis, attenuated the activation of hepatic stellate cells, reduced frequencies of Th9, Th17 and Th1 cells in spleen, and suppressed expression of IL-9, IL-17A, IFN-γ, TGF-β1, IL-6, IL-4 and TNF-α in plasma and liver respectively. Our data suggest a deleterious role of Th9/IL-9 in increasing hepatic fibrosis and exacerbating disease endpoints, indicating that Th9/IL9 based immunotherapy may be a promising approach for treating hepatic fibrosis.
Figures





References
-
- Karlmark K. R. et al. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol 2, 233–242 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

