Featured Article: Differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells
- PMID: 26729042
- PMCID: PMC4950326
- DOI: 10.1177/1535370215622584
Featured Article: Differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells
Abstract
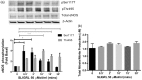
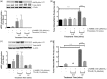
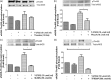
Protease-activated receptors have been shown to regulate endothelial nitric oxide synthase through the phosphorylation of specific sites on the enzyme. It has been established that PAR-2 activation phosphorylates eNOS-Ser-1177 and leads to the production of the potent vasodilator nitric oxide, while PAR-1 activation phosphorylates eNOS-Thr-495 and decreases nitric oxide production in human umbilical vein endothelial cells. In this study, we hypothesize a differential coupling of protease-activated receptors to the signaling pathways that regulates endothelial nitric oxide synthase and nitric oxide production in primary adult human coronary artery endothelial cells. Using Western Blot analysis, we showed that thrombin and the PAR-1 activating peptide, TFLLR, lead to the phosphorylation of eNOS-Ser-1177 in human coronary artery endothelial cells, which was blocked by SCH-79797 (SCH), a PAR-1 inhibitor. Using the nitrate/nitrite assay, we also demonstrated that the thrombin- and TFLLR-induced production of nitric oxide was inhibited by SCH and L-NAME, a NOS inhibitor. In addition, we observed that TFLLR, unlike thrombin, significantly phosphorylated eNOS-Thr-495, which may explain the observed delay in nitric oxide production in comparison to that of thrombin. Activation of PAR-2 by SLIGRL, a PAR-2 specific ligand, leads to dual phosphorylation of both catalytic sites but primarily regulated eNOS-Thr-495 phosphorylation with no change in nitric oxide production in human coronary artery endothelial cells. PAR-3, known as the non-signaling receptor, was activated by TFRGAP, a PAR-3 mimicking peptide, and significantly induced the phosphorylation of eNOS-Thr-495 with minimal phosphorylation of eNOS-Ser-1177 with no change in nitric oxide production. In addition, we confirmed that PAR-mediated eNOS-Ser-1177 phosphorylation was Ca(2+)-dependent using the Ca(2+) chelator, BAPTA, while eNOS-Thr-495 phosphorylation was mediated via Rho kinase using the ROCK inhibitor, Y-27632, suggesting protease-activated receptor coupling to Gq and G12/13, respectively. These data suggest a vascular bed specific differential coupling of protease-activated receptors to the signaling pathways that regulate endothelial nitric oxide synthase and nitric oxide production that may be responsible for endothelial dysfunction associated with cardiovascular disease.
Keywords: Endothelial nitric-oxide synthase; endothelial dysfunction; nitric oxide; protease-activated receptors; thrombin.
© 2016 by the Society for Experimental Biology and Medicine.
Figures





Similar articles
-
Role of protease-activated receptor-1 in endothelial nitric oxide synthase-Thr495 phosphorylation.Exp Biol Med (Maywood). 2009 Feb;234(2):132-9. doi: 10.3181/0807-RM-233. Epub 2008 Dec 8. Exp Biol Med (Maywood). 2009. PMID: 19064940
-
Distinct roles of protease-activated receptors in signal transduction regulation of endothelial nitric oxide synthase.Hypertension. 2009 Feb;53(2):182-8. doi: 10.1161/HYPERTENSIONAHA.108.125229. Epub 2008 Dec 8. Hypertension. 2009. PMID: 19064814 Free PMC article.
-
Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase.Circ Res. 2001 Sep 28;89(7):583-90. doi: 10.1161/hh1901.097084. Circ Res. 2001. PMID: 11577023
-
Endothelial caveolar subcellular domain regulation of endothelial nitric oxide synthase.Clin Exp Pharmacol Physiol. 2013 Nov;40(11):753-64. doi: 10.1111/1440-1681.12136. Clin Exp Pharmacol Physiol. 2013. PMID: 23745825 Free PMC article. Review.
-
eNOS activation and NO function: pregnancy adaptive programming of capacitative entry responses alters nitric oxide (NO) output in vascular endothelium--new insights into eNOS regulation through adaptive cell signaling.J Endocrinol. 2011 Sep;210(3):243-58. doi: 10.1530/JOE-11-0053. Epub 2011 May 9. J Endocrinol. 2011. PMID: 21555345 Free PMC article. Review.
Cited by
-
PAR2 activation in the dura causes acute behavioral responses and priming to glyceryl trinitrate in a mouse migraine model.J Headache Pain. 2023 Apr 19;24(1):42. doi: 10.1186/s10194-023-01574-5. J Headache Pain. 2023. PMID: 37072694 Free PMC article.
-
Betulinic acid‑induced expression of nicotinamide adenine dinucleotide phosphate‑diaphorase in the immune organs of mice: A possible role of nitric oxide in immunomodulation.Mol Med Rep. 2018 Feb;17(2):3035-3041. doi: 10.3892/mmr.2017.8262. Epub 2017 Dec 12. Mol Med Rep. 2018. PMID: 29257292 Free PMC article.
-
Protective role of protease-activated receptor-2 in anaphylaxis model mice.PLoS One. 2024 Apr 18;19(4):e0283915. doi: 10.1371/journal.pone.0283915. eCollection 2024. PLoS One. 2024. PMID: 38635782 Free PMC article.
-
Thrombin in Pregnancy and Preeclampsia: Expression, Localization, and Vasoactivity in Brain and Microvessels From Rats.J Cardiovasc Pharmacol. 2024 Aug 1;84(2):250-260. doi: 10.1097/FJC.0000000000001579. J Cardiovasc Pharmacol. 2024. PMID: 38922586 Free PMC article.
-
Use of Computational Biochemistry for Elucidating Molecular Mechanisms of Nitric Oxide Synthase.Comput Struct Biotechnol J. 2019 Mar 23;17:415-429. doi: 10.1016/j.csbj.2019.03.011. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 30996821 Free PMC article. Review.
References
-
- Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014; 64: 924–8. - PubMed
-
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000; 407: 258–64. - PubMed
-
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 2001; 53: 245–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

