Featured Article: Differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells
- PMID: 26729042
- PMCID: PMC4950326
- DOI: 10.1177/1535370215622584
Featured Article: Differential regulation of endothelial nitric oxide synthase phosphorylation by protease-activated receptors in adult human endothelial cells
Abstract
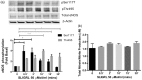
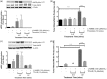
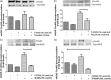
Protease-activated receptors have been shown to regulate endothelial nitric oxide synthase through the phosphorylation of specific sites on the enzyme. It has been established that PAR-2 activation phosphorylates eNOS-Ser-1177 and leads to the production of the potent vasodilator nitric oxide, while PAR-1 activation phosphorylates eNOS-Thr-495 and decreases nitric oxide production in human umbilical vein endothelial cells. In this study, we hypothesize a differential coupling of protease-activated receptors to the signaling pathways that regulates endothelial nitric oxide synthase and nitric oxide production in primary adult human coronary artery endothelial cells. Using Western Blot analysis, we showed that thrombin and the PAR-1 activating peptide, TFLLR, lead to the phosphorylation of eNOS-Ser-1177 in human coronary artery endothelial cells, which was blocked by SCH-79797 (SCH), a PAR-1 inhibitor. Using the nitrate/nitrite assay, we also demonstrated that the thrombin- and TFLLR-induced production of nitric oxide was inhibited by SCH and L-NAME, a NOS inhibitor. In addition, we observed that TFLLR, unlike thrombin, significantly phosphorylated eNOS-Thr-495, which may explain the observed delay in nitric oxide production in comparison to that of thrombin. Activation of PAR-2 by SLIGRL, a PAR-2 specific ligand, leads to dual phosphorylation of both catalytic sites but primarily regulated eNOS-Thr-495 phosphorylation with no change in nitric oxide production in human coronary artery endothelial cells. PAR-3, known as the non-signaling receptor, was activated by TFRGAP, a PAR-3 mimicking peptide, and significantly induced the phosphorylation of eNOS-Thr-495 with minimal phosphorylation of eNOS-Ser-1177 with no change in nitric oxide production. In addition, we confirmed that PAR-mediated eNOS-Ser-1177 phosphorylation was Ca(2+)-dependent using the Ca(2+) chelator, BAPTA, while eNOS-Thr-495 phosphorylation was mediated via Rho kinase using the ROCK inhibitor, Y-27632, suggesting protease-activated receptor coupling to Gq and G12/13, respectively. These data suggest a vascular bed specific differential coupling of protease-activated receptors to the signaling pathways that regulate endothelial nitric oxide synthase and nitric oxide production that may be responsible for endothelial dysfunction associated with cardiovascular disease.
Keywords: Endothelial nitric-oxide synthase; endothelial dysfunction; nitric oxide; protease-activated receptors; thrombin.
© 2016 by the Society for Experimental Biology and Medicine.
Figures





References
-
- Brandes RP. Endothelial dysfunction and hypertension. Hypertension 2014; 64: 924–8. - PubMed
-
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature 2000; 407: 258–64. - PubMed
-
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev 2001; 53: 245–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

