Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations
- PMID: 26729076
- PMCID: PMC6274182
- DOI: 10.3390/molecules21010044
Site-Selective Ribosylation of Fluorescent Nucleobase Analogs Using Purine-Nucleoside Phosphorylase as a Catalyst: Effects of Point Mutations
Abstract
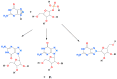
Enzymatic ribosylation of fluorescent 8-azapurine derivatives, like 8-azaguanine and 2,6-diamino-8-azapurine, with purine-nucleoside phosphorylase (PNP) as a catalyst, leads to N9, N8, and N7-ribosides. The final proportion of the products may be modulated by point mutations in the enzyme active site. As an example, ribosylation of the latter substrate by wild-type calf PNP gives N7- and N8-ribosides, while the N243D mutant directs the ribosyl substitution at N9- and N7-positions. The same mutant allows synthesis of the fluorescent N7-β-d-ribosyl-8-azaguanine. The mutated form of the E. coli PNP, D204N, can be utilized to obtain non-typical ribosides of 8-azaadenine and 2,6-diamino-8-azapurine as well. The N7- and N8-ribosides of the 8-azapurines can be analytically useful, as illustrated by N7-β-d-ribosyl-2,6-diamino-8-azapurine, which is a good fluorogenic substrate for mammalian forms of PNP, including human blood PNP, while the N8-riboside is selective to the E. coli enzyme.
Keywords: 8-azapurines; enzymatic ribosylation; fluorescent nucleosides; purine nucleoside phosphorylase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Chemo-Enzymatic Generation of Highly Fluorescent Nucleoside Analogs Using Purine-Nucleoside Phosphorylase.Biomolecules. 2024 Jun 14;14(6):701. doi: 10.3390/biom14060701. Biomolecules. 2024. PMID: 38927104 Free PMC article. Review.
-
Enzymatic synthesis of highly fluorescent 8-azapurine ribosides using a purine nucleoside phosphorylase reverse reaction: variable ribosylation sites.Molecules. 2013 Oct 11;18(10):12587-98. doi: 10.3390/molecules181012587. Molecules. 2013. PMID: 24126376 Free PMC article.
-
Two fluorogenic substrates for purine nucleoside phosphorylase, selective for mammalian and bacterial forms of the enzyme.Anal Biochem. 2014 Feb 1;446:25-7. doi: 10.1016/j.ab.2013.10.017. Epub 2013 Oct 17. Anal Biochem. 2014. PMID: 24140360
-
Tricyclic nitrogen base 1,N6-ethenoadenine and its ribosides as substrates for purine-nucleoside phosphorylases: Spectroscopic and kinetic studies.Nucleosides Nucleotides Nucleic Acids. 2018 Feb 1;37(2):89-101. doi: 10.1080/15257770.2017.1419255. Epub 2018 Jan 29. Nucleosides Nucleotides Nucleic Acids. 2018. PMID: 29376769
-
[Purine nucleoside phosphorylase].Biomed Khim. 2013 Sep-Oct;59(5):483-97. doi: 10.18097/pbmc20135905483. Biomed Khim. 2013. PMID: 24479338 Review. Russian.
Cited by
-
Pharmacological and Predicted Activities of Natural Azo Compounds.Nat Prod Bioprospect. 2017 Feb;7(1):151-169. doi: 10.1007/s13659-016-0117-3. Epub 2017 Jan 4. Nat Prod Bioprospect. 2017. PMID: 28054247 Free PMC article.
-
Tricyclic Nucleobase Analogs and Their Ribosides as Substrates and Inhibitors of Purine-Nucleoside Phosphorylases III. Aminopurine Derivatives.Molecules. 2020 Feb 5;25(3):681. doi: 10.3390/molecules25030681. Molecules. 2020. PMID: 32033464 Free PMC article.
-
Tri-Cyclic Nucleobase Analogs and their Ribosides as Substrates of Purine-Nucleoside Phosphorylases. II Guanine and Isoguanine Derivatives.Molecules. 2019 Apr 16;24(8):1493. doi: 10.3390/molecules24081493. Molecules. 2019. PMID: 30995785 Free PMC article.
-
Chemo-Enzymatic Generation of Highly Fluorescent Nucleoside Analogs Using Purine-Nucleoside Phosphorylase.Biomolecules. 2024 Jun 14;14(6):701. doi: 10.3390/biom14060701. Biomolecules. 2024. PMID: 38927104 Free PMC article. Review.
-
Enzymatic Transglycosylation Features in Synthesis of 8-Aza-7-Deazapurine Fleximer Nucleosides by Recombinant E. coli PNP: Synthesis and Structure Determination of Minor Products.Biomolecules. 2024 Jul 4;14(7):798. doi: 10.3390/biom14070798. Biomolecules. 2024. PMID: 39062512 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

