The Prodrug Approach: A Successful Tool for Improving Drug Solubility
- PMID: 26729077
- PMCID: PMC6273601
- DOI: 10.3390/molecules21010042
The Prodrug Approach: A Successful Tool for Improving Drug Solubility
Abstract
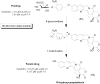
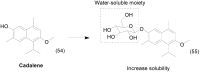
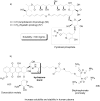
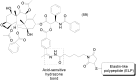
Prodrug design is a widely known molecular modification strategy that aims to optimize the physicochemical and pharmacological properties of drugs to improve their solubility and pharmacokinetic features and decrease their toxicity. A lack of solubility is one of the main obstacles to drug development. This review aims to describe recent advances in the improvement of solubility via the prodrug approach. The main chemical carriers and examples of successful strategies will be discussed, highlighting the advances of this field in the last ten years.
Keywords: molecular modification; prodrug; solubility; solubility of prodrugs; water-soluble prodrugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
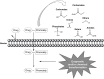
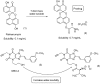
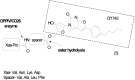
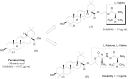
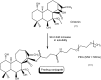
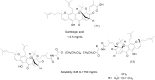

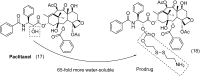

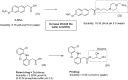
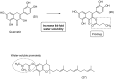

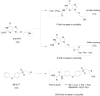
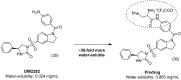

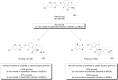
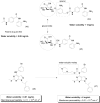
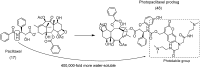
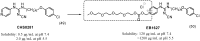
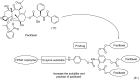


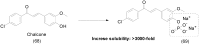
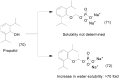
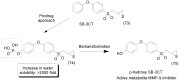

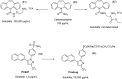

References
-
- Abu-jaish A., Jumaa S., Karaman R. Prodrug Overview. In: Karaman R., editor. Prodrugs Design: A New Era. Nova Publisher; Hauppauge, NY, USA: 2014. pp. 77–102.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

