Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells
- PMID: 26729093
- PMCID: PMC4730286
- DOI: 10.3390/ijms17010041
Alisertib Induces Cell Cycle Arrest, Apoptosis, Autophagy and Suppresses EMT in HT29 and Caco-2 Cells
Abstract
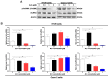
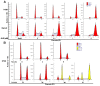
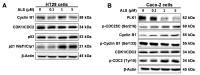
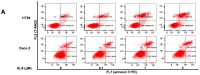
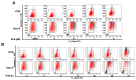
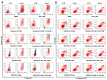
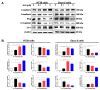
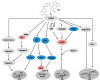
Colorectal cancer (CRC) is one of the most common malignancies worldwide with substantial mortality and morbidity. Alisertib (ALS) is a selective Aurora kinase A (AURKA) inhibitor with unclear effect and molecular interactome on CRC. This study aimed to evaluate the molecular interactome and anticancer effect of ALS and explore the underlying mechanisms in HT29 and Caco-2 cells. ALS markedly arrested cells in G₂/M phase in both cell lines, accompanied by remarkable alterations in the expression level of key cell cycle regulators. ALS induced apoptosis in HT29 and Caco-2 cells through mitochondrial and death receptor pathways. ALS also induced autophagy in HT29 and Caco-2 cells, with the suppression of phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR), but activation of 5' AMP-activated protein kinase (AMPK) signaling pathways. There was a differential modulating effect of ALS on p38 MAPK signaling pathway in both cell lines. Moreover, induction or inhibition of autophagy modulated basal and ALS-induced apoptosis in both cell lines. ALS potently suppressed epithelial to mesenchymal transition (EMT) in HT29 and Caco-2 cells. Collectively, it suggests that induction of cell cycle arrest, promotion of apoptosis and autophagy, and suppression of EMT involving mitochondrial, death receptor, PI3K/Akt/mTOR, p38 MAPK, and AMPK signaling pathways contribute to the cancer cell killing effect of ALS on CRC cells.
Keywords: EMT; alisertib; cell cycle; colorectal cancer; programmed cell death.
Figures






References
-
- Society A.C. Cancer facts and figures. [(accessed on 16 May 2013)]. Available online: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

