Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress
- PMID: 26729151
- PMCID: PMC4730479
- DOI: 10.3390/ijerph13010088
Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress
Abstract
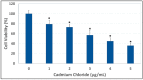
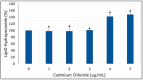
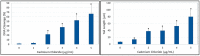
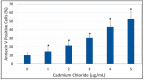
Cadmium is a heavy metal that has been shown to cause its toxicity in humans and animals. Many documented studies have shown that cadmium produces various genotoxic effects such as DNA damage and chromosomal aberrations. Ailments such as bone disease, renal damage, and several forms of cancer are attributed to overexposure to cadmium. Although there have been numerous studies examining the effects of cadmium in animal models and a few case studies involving communities where cadmium contamination has occurred, its molecular mechanisms of action are not fully elucidated. In this research, we hypothesized that oxidative stress plays a key role in cadmium chloride-induced toxicity, DNA damage, and apoptosis of human liver carcinoma (HepG₂) cells. To test our hypothesis, cell viability was determined by MTT assay. Lipid hydroperoxide content stress was estimated by lipid peroxidation assay. Genotoxic damage was tested by the means of alkaline single cell gel electrophoresis (Comet) assay. Cell apoptosis was measured by flow cytometry assessment (Annexin-V/PI assay). The result of MTT assay indicated that cadmium chloride induces toxicity to HepG₂ cells in a concentration-dependent manner, showing a 48 hr-LD50 of 3.6 µg/mL. Data generated from lipid peroxidation assay resulted in a significant (p < 0.05) increase of hydroperoxide production, specifically at the highest concentration tested. Data obtained from the Comet assay indicated that cadmium chloride causes DNA damage in HepG₂ cells in a concentration-dependent manner. A strong concentration-response relationship (p < 0.05) was recorded between annexin V positive cells and cadmium chloride exposure. In summary, these in vitro studies provide clear evidence that cadmium chloride induces oxidative stress, DNA damage, and programmed cell death in human liver carcinoma (HepG₂) cells.
Keywords: DNA damage; HepG2 cells; apoptosis; cadmium chloride; cytotoxicity; oxidative stress.
Figures


References
-
- International Agency for Research on Cancer (IARC) Monographs-Cadmium. International Agency for Research on Cancer (IARC); Lyon, France: 1993.
-
- Kasuya M., Teranishi H., Aoshima K., Katoh T., Horiguchi H., Morikawa Y. Water pollution by cadmium and the onset of Itai-itai disease. Water Sci. Technol. 2000;25:149–156.
-
- Jarup L., Berglund M., Elinder C., Nordberg G., Vahteram M. Health effects of cadmium exposure-a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;1:1–52. - PubMed
-
- Jin T., Lu J., Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothioneins. Neurotoxicology. 1998;19:529–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

