Tau Biology and Tau-Directed Therapies for Alzheimer's Disease
- PMID: 26729186
- PMCID: PMC4757605
- DOI: 10.1007/s40265-015-0529-0
Tau Biology and Tau-Directed Therapies for Alzheimer's Disease
Abstract
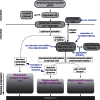
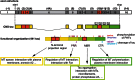
Alzheimer's disease (AD) is characterised by a progressive loss of cognitive functions. Histopathologically, AD is defined by the presence of extracellular amyloid plaques containing Aβ and intracellular neurofibrillary tangles composed of hyperphosphorylated tau proteins. According to the now well-accepted amyloid cascade hypothesis is the Aβ pathology the primary driving force of AD pathogenesis, which then induces changes in tau protein leading to a neurodegenerative cascade during the progression of disease. Since many earlier drug trials aiming at preventing Aβ pathology failed to demonstrate efficacy, tau and microtubules have come into focus as prominent downstream targets. The article aims to develop the current concept of the involvement of tau in the neurodegenerative triad of synaptic loss, cell death and dendritic simplification. The function of tau as a microtubule-associated protein and versatile interaction partner will then be introduced and the rationale and progress of current tau-directed therapy will be discussed in the biological context.
Figures
References
-
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/WNL.43.8.1467. - DOI - PubMed
-
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

