Claudin-21 Has a Paracellular Channel Role at Tight Junctions
- PMID: 26729464
- PMCID: PMC4810463
- DOI: 10.1128/MCB.00758-15
Claudin-21 Has a Paracellular Channel Role at Tight Junctions
Abstract
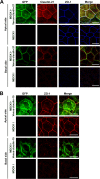
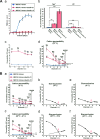
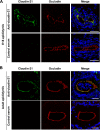
Claudin protein family members, of which there are at least 27 in humans and mice, polymerize to form tight junctions (TJs) between epithelial cells, in a tissue- and developmental stage-specific manner. Claudins have a paracellular barrier function. In addition, certain claudins function as paracellular channels for small ions and/or solutes by forming selective pores at the TJs, although the specific claudins involved and their functional mechanisms are still in question. Here we show for the first time that claudin-21, which is more highly expressed in the embryonic than the postnatal stages, acts as a paracellular channel for small cations, such as Na(+), similar to the typical channel-type claudins claudin-2 and -15. Claudin-21 also allows the paracellular passage of larger solutes. Our findings suggest that claudin-21-based TJs allow the passage of small and larger solutes by both paracellular channel-based and some additional mechanisms.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Site-specific distribution of claudin-based paracellular channels with roles in biological fluid flow and metabolism.Ann N Y Acad Sci. 2017 Oct;1405(1):44-52. doi: 10.1111/nyas.13438. Epub 2017 Sep 4. Ann N Y Acad Sci. 2017. PMID: 28869648 Review.
-
Tight junctions of the proximal tubule and their channel proteins.Pflugers Arch. 2017 Aug;469(7-8):877-887. doi: 10.1007/s00424-017-2001-3. Epub 2017 Jun 9. Pflugers Arch. 2017. PMID: 28600680 Review.
-
The Claudins: From Tight Junctions to Biological Systems.Trends Biochem Sci. 2019 Feb;44(2):141-152. doi: 10.1016/j.tibs.2018.09.008. Epub 2018 Oct 25. Trends Biochem Sci. 2019. PMID: 30665499 Review.
-
Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture.Am J Physiol Cell Physiol. 2003 Jun;284(6):C1346-54. doi: 10.1152/ajpcell.00547.2002. Epub 2003 Apr 16. Am J Physiol Cell Physiol. 2003. PMID: 12700140
-
The coculture method to examine interactions between claudin isoforms in tight junction-free HEK293 cells and tight junction-bearing MDCK II cells.Methods Mol Biol. 2011;762:101-14. doi: 10.1007/978-1-61779-185-7_8. Methods Mol Biol. 2011. PMID: 21717352
Cited by
-
Multiscale modelling of claudin-based assemblies: A magnifying glass for novel structures of biological interfaces.Comput Struct Biotechnol J. 2022 Oct 28;20:5984-6010. doi: 10.1016/j.csbj.2022.10.038. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36382184 Free PMC article. Review.
-
MicroRNAs in intestinal barrier function, inflammatory bowel disease and related cancers-their effects and therapeutic potentials.Curr Opin Pharmacol. 2017 Dec;37:142-150. doi: 10.1016/j.coph.2017.10.010. Epub 2017 Nov 15. Curr Opin Pharmacol. 2017. PMID: 29154194 Free PMC article. Review.
-
Claudin 1, 4, 6 and 18 isoform 2 as targets for the treatment of cancer (Review).Int J Mol Med. 2024 Nov;54(5):100. doi: 10.3892/ijmm.2024.5424. Epub 2024 Sep 20. Int J Mol Med. 2024. PMID: 39301632 Free PMC article. Review.
-
A Novel Claudinopathy Based on Claudin-10 Mutations.Int J Mol Sci. 2019 Oct 30;20(21):5396. doi: 10.3390/ijms20215396. Int J Mol Sci. 2019. PMID: 31671507 Free PMC article. Review.
-
The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies.Int J Mol Sci. 2024 May 21;25(11):5601. doi: 10.3390/ijms25115601. Int J Mol Sci. 2024. PMID: 38891789 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
