Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function
- PMID: 26729601
- PMCID: PMC4728429
- DOI: 10.1038/ncomms10184
Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function
Abstract
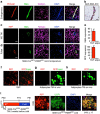
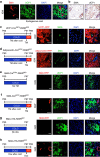
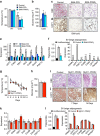
Cold temperatures induce formation of beige adipocytes, which convert glucose and fatty acids to heat, and may increase energy expenditure, reduce adiposity and lower blood glucose. This therapeutic potential is unrealized, hindered by a dearth of genetic tools to fate map, track and manipulate beige progenitors and 'beiging'. Here we examined 12 Cre/inducible Cre mouse strains that mark adipocyte, muscle and mural lineages, three proposed beige origins. Among these mouse strains, only those that marked perivascular mural cells tracked the cold-induced beige lineage. Two SMA-based strains, SMA-Cre(ERT2) and SMA-rtTA, fate mapped into the majority of cold-induced beige adipocytes and SMA-marked progenitors appeared essential for beiging. Disruption of the potential of the SMA-tracked progenitors to form beige adipocytes was accompanied by an inability to maintain body temperature and by hyperglycaemia. Thus, SMA-engineered mice may be useful to track and manipulate beige progenitors, beige adipocyte formation and function.
Conflict of interest statement
J.M.G. is a co-founder and shareholder of Reata Pharmaceuticals. D.C.B. and Y.J. declare no competing financial interests.
Figures
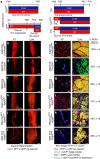
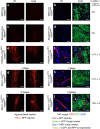
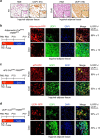
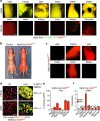

References
-
- Harms M. & Seale P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013). - PubMed
-
- Yoneshiro T. et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 19, 13–16 (2011). - PubMed
-
- Boucher P., Gotthardt M., Li W. P., Anderson R. G. & Herz J. LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300, 329–332 (2003). - PubMed
-
- Lidell M. E., Betz M. J. & Enerback S. Brown adipose tissue and its therapeutic potential. J. Intern. Med. 276, 364–377 (2014). - PubMed
-
- Barbatelli G. et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

