Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity
- PMID: 26729873
- PMCID: PMC4760822
- DOI: 10.1073/pnas.1516979113
Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity
Abstract
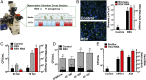
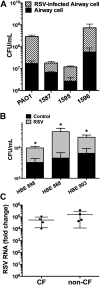
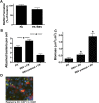
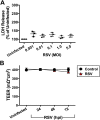
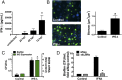
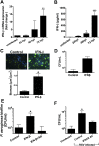
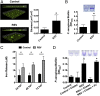
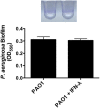
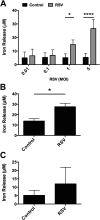
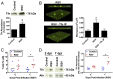
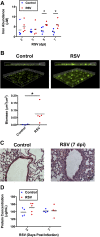
Clinical observations link respiratory virus infection and Pseudomonas aeruginosa colonization in chronic lung disease, including cystic fibrosis (CF) and chronic obstructive pulmonary disease. The development of P. aeruginosa into highly antibiotic-resistant biofilm communities promotes airway colonization and accounts for disease progression in patients. Although clinical studies show a strong correlation between CF patients' acquisition of chronic P. aeruginosa infections and respiratory virus infection, little is known about the mechanism by which chronic P. aeruginosa infections are initiated in the host. Using a coculture model to study the formation of bacterial biofilm formation associated with the airway epithelium, we show that respiratory viral infections and the induction of antiviral interferons promote robust secondary P. aeruginosa biofilm formation. We report that the induction of antiviral IFN signaling in response to respiratory syncytial virus (RSV) infection induces bacterial biofilm formation through a mechanism of dysregulated iron homeostasis of the airway epithelium. Moreover, increased apical release of the host iron-binding protein transferrin during RSV infection promotes P. aeruginosa biofilm development in vitro and in vivo. Thus, nutritional immunity pathways that are disrupted during respiratory viral infection create an environment that favors secondary bacterial infection and may provide previously unidentified targets to combat bacterial biofilm formation.
Keywords: Pseudomonas aeruginosa; biofilm; cystic fibrosis; nutritional immunity; respiratory syncytial virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Cystic fibrosis and the war for iron at the host-pathogen battlefront.Proc Natl Acad Sci U S A. 2016 Feb 9;113(6):1480-2. doi: 10.1073/pnas.1525101113. Epub 2016 Jan 22. Proc Natl Acad Sci U S A. 2016. PMID: 26802119 Free PMC article. No abstract available.
References
-
- Suárez-Arrabal MC, et al. Nasopharyngeal bacterial burden and antibiotics: Influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Infect. 2015;71(4):458–469. - PubMed
-
- Varkey JB, Varkey B. Viral infections in patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2008;14(2):89–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

