Nanostructured ultra-thin patches for ultrasound-modulated delivery of anti-restenotic drug
- PMID: 26730191
- PMCID: PMC4694686
- DOI: 10.2147/IJN.S92031
Nanostructured ultra-thin patches for ultrasound-modulated delivery of anti-restenotic drug
Abstract
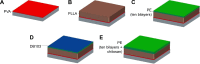
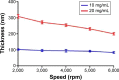
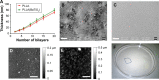
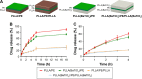
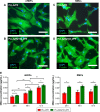
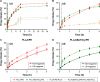
This work aims to demonstrate the possibility to fabricate ultra-thin polymeric films loaded with an anti-restenotic drug and capable of tunable drug release kinetics for the local treatment of restenosis. Vascular nanopatches are composed of a poly(lactic acid) supporting membrane (thickness: ~250 nm) on which 20 polyelectrolyte bilayers (overall thickness: ~70 nm) are alternatively deposited. The anti-restenotic drug is embedded in the middle of the polyelectrolyte structure, and released by diffusion mechanisms. Nanofilm fabrication procedure and detailed morphological characterization are reported here. Barium titanate nanoparticles (showing piezoelectric properties) are included in the polymeric support and their role is investigated in terms of influence on nanofilm morphology, drug release kinetics, and cell response. Results show an efficient drug release from the polyelectrolyte structure in phosphate-buffered saline, and a clear antiproliferative effect on human smooth muscle cells, which are responsible for restenosis. In addition, preliminary evidences of ultrasound-mediated modulation of drug release kinetics are reported, thus evaluating the influence of barium titanate nanoparticles on the release mechanism. Such data were integrated with quantitative piezoelectric and thermal measurements. These results open new avenues for a fine control of local therapies based on smart responsive materials.
Keywords: barium titanate nanoparticles; drug delivery; layer-by-layer polyelectrolytes; micro/nanotherapeutic systems; restenosis; thin films; ultrasound.
Figures





References
-
- Nobuyoshi M, Kimura T, Ohishi H, et al. Restenosis after percutaneous transluminal coronary angioplasty: pathologic observations in 20 patients. J Am Coll Cardiol. 1991;17(2):433–439. - PubMed
-
- Chakhtoura EY, Hobson RW, II, Goldstein J, et al. In-stent restenosis after carotid angioplasty-stenting: incidence and management. J Vasc Surg. 2001;33(2):220–226. - PubMed
-
- Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Card. 2012;59(1):16–23. - PubMed
-
- Austin GE, Ratliff NB, Hollman J, Tabei S, Phillips DF. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985;6(2):369–375. - PubMed
-
- Zargham R. Preventing restenosis after angioplasty: a multistage approach. Clin Sci. 2008;114:257–264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

