Solvents and sustainable chemistry
- PMID: 26730217
- PMCID: PMC4685879
- DOI: 10.1098/rspa.2015.0502
Solvents and sustainable chemistry
Abstract
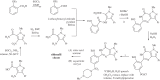
Solvents are widely recognized to be of great environmental concern. The reduction of their use is one of the most important aims of green chemistry. In addition to this, the appropriate selection of solvent for a process can greatly improve the sustainability of a chemical production process. There has also been extensive research into the application of so-called green solvents, such as ionic liquids and supercritical fluids. However, most examples of solvent technologies that give improved sustainability come from the application of well-established solvents. It is also apparent that the successful implementation of environmentally sustainable processes must be accompanied by improvements in commercial performance.
Keywords: green chemistry; process chemistry; solvent; sustainability; sustainable chemistry.
Figures









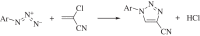


Similar articles
-
Green and Sustainable Solvents in Chemical Processes.Chem Rev. 2018 Jan 24;118(2):747-800. doi: 10.1021/acs.chemrev.7b00571. Epub 2018 Jan 4. Chem Rev. 2018. PMID: 29300087
-
Tailor-designed deep eutectic liquids as a sustainable extraction media: An alternative to ionic liquids.J Pharm Biomed Anal. 2019 Sep 10;174:324-329. doi: 10.1016/j.jpba.2019.05.059. Epub 2019 May 27. J Pharm Biomed Anal. 2019. PMID: 31195320 Review.
-
Using more environmentally friendly solvents and benign catalysts in performing conventional organic reactions.Curr Opin Drug Discov Devel. 2010;13(6):658-68. Curr Opin Drug Discov Devel. 2010. PMID: 21061229 Review.
-
Mini-review: green sustainable processes using supercritical fluid carbon dioxide.J Environ Sci (China). 2009;21(6):720-6. doi: 10.1016/s1001-0742(08)62330-x. J Environ Sci (China). 2009. PMID: 19803072 Review.
-
Ionic liquids and deep eutectic mixtures: sustainable solvents for extraction processes.ChemSusChem. 2014 Jul;7(7):1784-800. doi: 10.1002/cssc.201301192. Epub 2014 May 8. ChemSusChem. 2014. PMID: 24811900 Review.
Cited by
-
Detection Method and Common Characteristics of Waste Solvent from Semiconductor Industry.Molecules. 2023 Aug 10;28(16):5992. doi: 10.3390/molecules28165992. Molecules. 2023. PMID: 37630244 Free PMC article.
-
Inhibition Control by Continuous Extractive Fermentation Enhances De Novo 2-Phenylethanol Production by Yeast.Biotechnol Bioeng. 2025 Feb;122(2):287-297. doi: 10.1002/bit.28872. Epub 2024 Oct 25. Biotechnol Bioeng. 2025. PMID: 39460388 Free PMC article.
-
Searching for Solvents with an Increased Carbon Dioxide Solubility Using Multivariate Statistics.Molecules. 2020 Mar 5;25(5):1156. doi: 10.3390/molecules25051156. Molecules. 2020. PMID: 32150808 Free PMC article.
-
Spectrophotometric approach for deconvolving overlapped spectra of antihypertensive drug mixtures using UV detection: an eco-friendly method.BMC Chem. 2025 Jul 14;19(1):211. doi: 10.1186/s13065-025-01565-4. BMC Chem. 2025. PMID: 40660360 Free PMC article.
-
Diarylethene-Based Ionic Liquids: Synthesis and Photo-Driven Solution Properties.Int J Mol Sci. 2023 Feb 9;24(4):3533. doi: 10.3390/ijms24043533. Int J Mol Sci. 2023. PMID: 36834945 Free PMC article.
References
-
- Brundtland CG. 1987. Our common future. Oxford, UK: World Commission on Environment and Development, Oxford University Press.
-
- Lancaster M. 2002. Introduction to green chemistry. Cambridge, UK: Royal Society of Chemistry.
-
- Anastas PT, Warner JC. 1998. Green chemistry theory and practice. New York, NY: Oxford University Press.
-
- Anastas P, Eghbali N. 2010. Green chemistry: principles and practice. Chem. Soc. Rev. 39, 301–312. (doi:10.1039/B918763B) - DOI - PubMed
-
- Cséfalvay E, Akien GR, Qi L, Horváth IT. 2015. Definition and application of ethanol equivalent: sustainability performance metrics for biomass conversion to carbon-based fuels and chemicals. Catal. Today 239, 50–55. (doi:10.1016/j.cattod.2014.02.006) - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

