Characterization of dendritic morphology and neurotransmitter phenotype of thoracic descending propriospinal neurons after complete spinal cord transection and GDNF treatment
- PMID: 26730519
- PMCID: PMC4761305
- DOI: 10.1016/j.expneurol.2015.12.018
Characterization of dendritic morphology and neurotransmitter phenotype of thoracic descending propriospinal neurons after complete spinal cord transection and GDNF treatment
Abstract
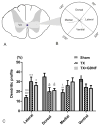
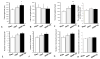
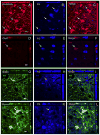
After spinal cord injury (SCI), poor regeneration of damaged axons of the central nervous system (CNS) causes limited functional recovery. This limited spontaneous functional recovery has been attributed, to a large extent, to the plasticity of propriospinal neurons, especially the descending propriospinal neurons (dPSNs). Compared with the supraspinal counterparts, dPSNs have displayed significantly greater regenerative capacity, which can be further enhanced by glial cell line-derived neurotrophic factor (GDNF). In the present study, we applied a G-mutated rabies virus (G-Rabies) co-expressing green fluorescence protein (GFP) to reveal Golgi-like dendritic morphology of dPSNs. We also investigated the neurotransmitters expressed by dPSNs after labeling with a retrograde tracer Fluoro-Gold (FG). dPSNs were examined in animals with sham injuries or complete spinal transections with or without GDNF treatment. Bilateral injections of G-Rabies and FG were made into the 2nd lumbar (L2) spinal cord at 3 days prior to a spinal cord transection performed at the 11th thoracic level (T11). The lesion gap was filled with Gelfoam containing either saline or GDNF in the injury groups. Four days post-injury, the rats were sacrificed for analysis. For those animals receiving G-rabies injection, the GFP signal in the T7-9 spinal cord was visualized via 2-photon microscopy. Dendritic morphology from stack images was traced and analyzed using a Neurolucida software. We found that dPSNs in sham injured animals had a predominantly dorsal-ventral distribution of dendrites. Transection injury resulted in alterations in the dendritic distribution with dorsal-ventral retraction and lateral-medial extension. Treatment with GDNF significantly increased the terminal dendritic length of dPSNs. The density of spine-like structures was increased after injury, and treatment with GDNF enhanced this effect. For the group receiving FG injections, immunohistochemistry for glutamate, choline acetyltransferase (ChAT), glycine, and GABA was performed in the T7-9 spinal cord. We show that the majority of FG retrogradely-labeled dPSNs were located in the Rexed Lamina VII. Over 90% of FG-labeled neurons were glutamatergic, with the other three neurotransmitters contributing less than 10% of the total. To our knowledge this is the first report describing the morphologic characteristics of dPSNs and their neurotransmitter expressions, as well as the dendritic response of dPSNs after transection injury and GDNF treatment.
Keywords: Dendrite; Descending propriospinal neuron; GDNF; Rabies virus; Spinal cord injury; Spine.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
No competing financial interests exist.
Figures


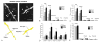
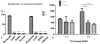
Similar articles
-
A neuroprotective role of glial cell line-derived neurotrophic factor following moderate spinal cord contusion injury.Exp Neurol. 2004 Oct;189(2):317-32. doi: 10.1016/j.expneurol.2004.05.033. Exp Neurol. 2004. PMID: 15380482
-
Restriction of axonal retraction and promotion of axonal regeneration by chronically injured neurons after intraspinal treatment with glial cell line-derived neurotrophic factor (GDNF).J Neurotrauma. 2003 Nov;20(11):1251-61. doi: 10.1089/089771503770802916. J Neurotrauma. 2003. PMID: 14651811
-
Regeneration of Propriospinal Axons in Rat Transected Spinal Cord Injury through a Growth-Promoting Pathway Constructed by Schwann Cells Overexpressing GDNF.Cells. 2024 Jul 8;13(13):1160. doi: 10.3390/cells13131160. Cells. 2024. PMID: 38995011 Free PMC article.
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury.Brain Res. 2015 Sep 4;1619:104-14. doi: 10.1016/j.brainres.2014.09.038. Epub 2014 Sep 26. Brain Res. 2015. PMID: 25257034 Free PMC article. Review.
Cited by
-
Protective Effects of Estradiol and Dihydrotestosterone following Spinal Cord Injury.J Neurotrauma. 2018 Mar 15;35(6):825-841. doi: 10.1089/neu.2017.5329. Epub 2018 Jan 11. J Neurotrauma. 2018. PMID: 29132243 Free PMC article.
-
Dual-Viral Transduction Utilizing Highly Efficient Retrograde Lentivirus Improves Labeling of Long Propriospinal Neurons.Front Neuroanat. 2021 Mar 22;15:635921. doi: 10.3389/fnana.2021.635921. eCollection 2021. Front Neuroanat. 2021. PMID: 33828464 Free PMC article.
-
Targeting Enolase in Reducing Secondary Damage in Acute Spinal Cord Injury in Rats.Neurochem Res. 2017 Oct;42(10):2777-2787. doi: 10.1007/s11064-017-2291-z. Epub 2017 May 15. Neurochem Res. 2017. PMID: 28508172 Free PMC article.
-
Propriospinal Neurons of L3-L4 Segments Involved in Control of the Rat External Urethral Sphincter.Neuroscience. 2020 Jan 15;425:12-28. doi: 10.1016/j.neuroscience.2019.11.013. Epub 2019 Nov 27. Neuroscience. 2020. PMID: 31785359 Free PMC article.
-
Mechanisms of Stem Cell Therapy in Spinal Cord Injuries.Cells. 2021 Oct 6;10(10):2676. doi: 10.3390/cells10102676. Cells. 2021. PMID: 34685655 Free PMC article. Review.
References
-
- Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Molecular and cellular neurosciences. 1999;13:313–325. - PubMed
-
- Alstermark B, Isa T. Premotoneuronal and direct corticomotoneuronal control in the cat and macaque monkey. Advances in experimental medicine and biology. 2002;508:281–297. - PubMed
-
- Alstermark B, Isa T, Pettersson LG, Sasaki S. The C3-C4 propriospinal system in the cat and monkey: a spinal pre-motoneuronal centre for voluntary motor control. Acta physiologica. 2007;189:123–140. - PubMed
-
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. 12. Interneurones which may mediate descending feed-forward inhibition and feed-back inhibition from the forelimb to C3-C4 propriospinal neurones. Experimental brain research. 1984;56:308–322. - PubMed
-
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annual review of neuroscience. 2007;30:79–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

