Disconnection and hyper-connectivity underlie reorganization after TBI: A rodent functional connectomic analysis
- PMID: 26730520
- PMCID: PMC4761291
- DOI: 10.1016/j.expneurol.2015.12.020
Disconnection and hyper-connectivity underlie reorganization after TBI: A rodent functional connectomic analysis
Abstract
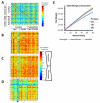
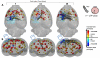
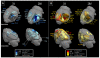
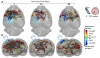
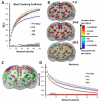
While past neuroimaging methods have contributed greatly to our understanding of brain function after traumatic brain injury (TBI), resting state functional MRI (rsfMRI) connectivity methods have more recently provided a far more unbiased approach with which to monitor brain circuitry compared to task-based approaches. However, current knowledge on the physiologic underpinnings of the correlated blood oxygen level dependent signal, and how changes in functional connectivity relate to reorganizational processes that occur following injury is limited. The degree and extent of this relationship remain to be determined in order that rsfMRI methods can be fully adapted for determining the optimal timing and type of rehabilitative interventions that can be used post-TBI to achieve the best outcome. Very few rsfMRI studies exist after experimental TBI and therefore we chose to acquire rsfMRI data before and at 7, 14 and 28 days after experimental TBI using a well-known, clinically-relevant, unilateral controlled cortical impact injury (CCI) adult rat model of TBI. This model was chosen since it has widespread axonal injury, a well-defined time-course of reorganization including spine, dendrite, axonal and cortical map changes, as well as spontaneous recovery of sensorimotor function by 28 d post-injury from which to interpret alterations in functional connectivity. Data were co-registered to a parcellated rat template to generate adjacency matrices for network analysis by graph theory. Making no assumptions about direction of change, we used two-tailed statistical analysis over multiple brain regions in a data-driven approach to access global and regional changes in network topology in order to assess brain connectivity in an unbiased way. Our main hypothesis was that deficits in functional connectivity would become apparent in regions known to be structurally altered or deficient in axonal connectivity in this model. The data show the loss of functional connectivity predicted by the structural deficits, not only within the primary sensorimotor injury site and pericontused regions, but the normally connected homotopic cortex, as well as subcortical regions, all of which persisted chronically. Especially novel in this study is the unanticipated finding of widespread increases in connection strength that dwarf both the degree and extent of the functional disconnections, and which persist chronically in some sensorimotor and subcortically connected regions. Exploratory global network analysis showed changes in network parameters indicative of possible acutely increased random connectivity and temporary reductions in modularity that were matched by local increases in connectedness and increased efficiency among more weakly connected regions. The global network parameters: shortest path-length, clustering coefficient and modularity that were most affected by trauma also scaled with the severity of injury, so that the corresponding regional measures were correlated to the injury severity most notably at 7 and 14 days and especially within, but not limited to, the contralateral cortex. These changes in functional network parameters are discussed in relation to the known time-course of physiologic and anatomic data that underlie structural and functional reorganization in this experiment model of TBI.
Keywords: Bold; Connectivity; Controlled cortical impact; Plasticity; Rat; Reorganization; Resting state fMRI.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Abdelnour F, Voss HU, Raj A. Network diffusion accurately models the relationship between structural and functional brain connectivity networks. NeuroImage. 2014;90:335–347. http://dx.doi.org/10.1016/j.neuroimage.2013.12.039. - DOI - PMC - PubMed
-
- de Arcangelis L, Herrmann HJ. Activity-dependent neuronal model on complex networks. Front. Physiol. 2012;3:1–9. http://dx.doi.org/10.3389/fphys.2012.00062 (MAR) - DOI - PMC - PubMed
-
- Benali A, Weiler E, Benali Y, et al. Excitation and inhibition jointly regulate cortical reorganization in adult rats. J. Neurosci. 2008;28:12284–12293. http://dx.doi.org/10.1523/JNEUROSCI.1952-08.2008. - DOI - PMC - PubMed
-
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34:537–541. - PubMed
-
- Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. 2008;10:P10008. http://dx.doi.org/10.1088/1742-5468/2008/10/P10008. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

