Tipranavir/Ritonavir (500/200 mg and 500/100 mg) Was Virologically Non-Inferior to Lopinavir/Ritonavir (400/100 mg) at Week 48 in Treatment-Naïve HIV-1-Infected Patients: A Randomized, Multinational, Multicenter Trial
- PMID: 26730818
- PMCID: PMC4701182
- DOI: 10.1371/journal.pone.0144917
Tipranavir/Ritonavir (500/200 mg and 500/100 mg) Was Virologically Non-Inferior to Lopinavir/Ritonavir (400/100 mg) at Week 48 in Treatment-Naïve HIV-1-Infected Patients: A Randomized, Multinational, Multicenter Trial
Abstract
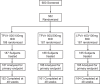
Ritonavir-boosted tipranavir (TPV/r) was evaluated as initial therapy in treatment-naïve HIV-1-infected patients because of its potency, unique resistance profile, and high genetic barrier. Trial 1182.33, an open-label, randomized trial, compared two TPV/r dose combinations versus ritonavir-boosted lopinavir (LPV/r). Eligible adults, who had no prior antiretroviral therapy were randomized to twice daily (BID) 500/100 mg TPV/r, 500/200 mg TPV/r, or 400/100 mg LPV/r. Each treatment group also received Tenofovir 300 mg + Lamivudine 300 mg QD. The primary endpoint was a confirmed viral load (VL) <50 copies/mL at week 48 without prior antiretroviral regimen changes. Primary analyses examined CD4-adjusted response rates for non-inferiority, using a 15% non-inferiority margin. At week 48, VL<50 copies/mL was 68.4%, 69.9%, and 72.4% in TPV/r100, TPV/r200, and LPV/r groups, respectively, and TPV/r groups showed non-inferiority to LPV/r. Discontinuation due to adverse events was higher in TPV/r100 (10.3%) and TPV/r200 (15.3%) recipients versus LPV/r (3.2%) recipients. The frequency of grade ≥3 transaminase elevations was higher in the TPV/r200 group than the other groups, leading to closure of this group. However, upon continued treatment or following re-introduction after treatment interruption, transaminase elevations returned to grade ≤2 in >65% of patients receiving either TPV/r200 or TPV/r100. The trial was subsequently discontinued; primary objectives were achieved and continuing TPV/r100 was less tolerable than standard of care for initial highly active antiretroviral therapy. All treatment groups had similar 48-week treatment responses. TPV/r100 and TPV/r200 regimens resulted in sustained treatment responses, which were non-inferior to LPV/r at 48 weeks. When compared with the LPV/r regimen and examined in the light of more current regimens, these TPV/r regimens do not appear to be the best options for treatment-naïve patients based on their safety profiles.
Conflict of interest statement
Figures
Similar articles
-
Effects of boosted tipranavir and lopinavir on body composition, insulin sensitivity and adipocytokines in antiretroviral-naive adults.AIDS. 2008 Nov 12;22(17):2313-21. doi: 10.1097/QAD.0b013e328315a7a5. AIDS. 2008. PMID: 18981770 Clinical Trial.
-
A lopinavir/ritonavir-based once-daily regimen results in better compliance and is non-inferior to a twice-daily regimen through 96 weeks.AIDS Res Hum Retroviruses. 2007 Dec;23(12):1505-14. doi: 10.1089/aid.2007.0107. AIDS Res Hum Retroviruses. 2007. PMID: 18160008 Clinical Trial.
-
Efficacy, safety and tolerability of tipranavir coadministered with ritonavir in HIV-1-infected children and adolescents.AIDS. 2008 Sep 12;22(14):1789-98. doi: 10.1097/QAD.0b013e32830c481b. AIDS. 2008. PMID: 18753862 Free PMC article. Clinical Trial.
-
Tipranavir: the first nonpeptidic protease inhibitor for the treatment of protease resistance.Clin Ther. 2007 Nov;29(11):2309-18. doi: 10.1016/j.clinthera.2007.11.007. Clin Ther. 2007. PMID: 18158073 Review.
-
Tipranavir: a ritonavir-boosted protease inhibitor.Drugs. 2005;65(12):1669-77; discussion 1678-9. doi: 10.2165/00003495-200565120-00005. Drugs. 2005. PMID: 16060700 Review.
Cited by
-
Multidomain drug delivery systems of β-casein micelles for the local oral co-administration of antiretroviral combinations.J Colloid Interface Sci. 2021 Jun 15;592:156-166. doi: 10.1016/j.jcis.2020.12.021. Epub 2021 Jan 1. J Colloid Interface Sci. 2021. PMID: 33652169 Free PMC article.
-
A target safety assessment of the potential toxicological risks of targeting plasmepsin IX/X for the treatment of malaria.Toxicol Res (Camb). 2021 Feb 15;10(2):203-213. doi: 10.1093/toxres/tfaa106. eCollection 2021 Mar. Toxicol Res (Camb). 2021. PMID: 33884171 Free PMC article. Review.
References
-
- Cahn P, Villacian J, Lazzarin A, Katlama C, Grinsztejn B, Arasteh K, et al. Ritonavir-boosted tipranavir demonstrates superior efficacy to ritonavir-boosted protease inhibitors in treatment-experienced HIV-infected patients: 24-week results of the RESIST-2 trial. Clin Infect Dis. 2006;43: 1347–1356. - PubMed
-
- Hicks CB, Cahn P, Cooper DA, Walmsley SL, Katlama C, Clotet B, et al. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet. 2006;368: 466–475. - PubMed
-
- Staszewski S, Morales-Ramirez J, Tashima KT, Skiest D, Stanford J, Stryker R, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. New England Journal of Medicine. 1999;341(25): 1865–1873. - PubMed
-
- Günthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Atiretroviral treatment of adult HIV infection 2014 –Recommendations of the International Antiviral society–USA Panel. Journal Amer Medical Assoc. 2014;312: 410–425. - PubMed
-
- Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. for ING114915 Study Team. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383: 2222–22231. 10.1016/S0140-6736(14)60084-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials