Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection
- PMID: 26731473
- PMCID: PMC4731186
- DOI: 10.1172/JCI84428
Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection
Abstract
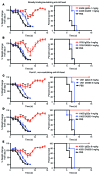
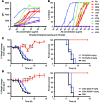
In vivo protection by antimicrobial neutralizing Abs can require the contribution of effector functions mediated by Fc-Fcγ receptor (Fc-FcγR) interactions for optimal efficacy. In influenza, broadly neutralizing anti-hemagglutinin (anti-HA) stalk mAbs require Fc-FcγR interactions to mediate in vivo protection, but strain-specific anti-HA head mAbs do not. Whether this rule applies only to anti-stalk Abs or is applicable to any broadly neutralizing Ab (bNAb) against influenza is unknown. Here, we characterized the contribution of Fc-FcγR interactions during in vivo protection for a panel of 13 anti-HA mAbs, including bNAbs and non-neutralizing Abs, against both the stalk and head domains. All classes of broadly binding anti-HA mAbs required Fc-FcγR interactions to provide protection in vivo, including those mAbs that bind the HA head and those that do not neutralize virus in vitro. Further, a broadly neutralizing anti-neuraminidase (anti-NA) mAb also required FcγRs to provide protection in vivo, but a strain-specific anti-NA mAb did not. Thus, these findings suggest that the breadth of reactivity of anti-influenza Abs, regardless of their epitope, necessitates interactions with FcγRs on effector cell populations to mediate in vivo protection. These findings will guide the design of antiviral Ab therapeutics and inform vaccine design to elicit Abs with optimal binding properties and effector functions.
Figures

References
-
- WHO Influenza (Seasonal) Fact sheet 211. [March 1, 2014]; [November 30, 2015];WHO Web site. http://www.who.int/mediacentre/factsheets/fs211/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

