A role for airway taste receptor modulation in the treatment of upper respiratory infections
- PMID: 26731661
- PMCID: PMC4830394
- DOI: 10.1586/17476348.2016.1135742
A role for airway taste receptor modulation in the treatment of upper respiratory infections
Abstract
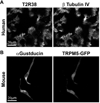
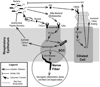
Taste receptors, initially identified in the oral epithelium, have since been shown to be widely distributed, being found in the upper and lower respiratory tracts, gastrointestinal epithelium, thyroid, and brain. The presence of taste receptors in the nasal epithelium has led to the discovery of their role in innate immunity, defending the paranasal sinuses against pathogens. This article addresses the current paradigm for understanding the role of extraoral taste receptors, specifically the T2R38 bitter taste receptor and the T1R2+3 sweet taste receptor, in respiratory innate defenses and presents evidence for the use of these and other taste receptors as therapeutic targets in the management of chronic rhinosinusitis. Future studies should focus on understanding the polymorphisms of taste receptors beyond T2R38 to fully elucidate their potential therapeutic use and lay the groundwork for their modulation in a clinical setting to decrease the health impact and economic burden of upper respiratory disease.
Keywords: Airway physiology; TAS2R; chronic rhinosinusitis; epithelial biology; host-pathogen interactions; innate immunity; taste receptors; upper respiratory disease.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. 2014;10(260):1–161. - PubMed
-
- Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103(3 Pt 1):408–414. - PubMed
-
- Genoway KA, Philpott CM, Javer AR. Pathogen yield and antimicrobial resistance patterns of chronic rhinosinusitis patients presenting to a tertiary rhinology centre. J Otolaryngol Head Neck Surg. 2011;40(3):232–237. - PubMed
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113(1):104–109. - PubMed
-
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34(5):447–454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
