Direct and indirect genetic effects of sex-specific mitonuclear epistasis on reproductive ageing
- PMID: 26732015
- PMCID: PMC4751623
- DOI: 10.1038/hdy.2015.112
Direct and indirect genetic effects of sex-specific mitonuclear epistasis on reproductive ageing
Abstract
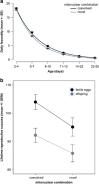
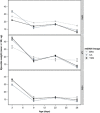
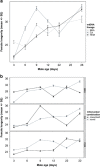
Mitochondria are involved in ageing and their function requires coordinated action of both mitochondrial and nuclear genes. Epistasis between the two genomes can influence lifespan but whether this also holds for reproductive senescence is unclear. Maternal inheritance of mitochondria predicts sex differences in the efficacy of selection on mitonuclear genotypes that should result in differences between females and males in mitochondrial genetic effects. Mitonuclear genotype of a focal individual may also indirectly affect trait expression in the mating partner. We tested these predictions in the seed beetle Callosobruchus maculatus, using introgression lines harbouring distinct mitonuclear genotypes. Our results reveal both direct and indirect sex-specific effects of mitonuclear epistasis on reproductive ageing. Females harbouring coadapted mitonuclear genotypes showed higher lifetime fecundity due to slower senescence relative to novel mitonuclear combinations. We found no evidence for mitonuclear coadaptation in males. Mitonuclear epistasis not only affected age-specific ejaculate weight, but also influenced male age-dependent indirect effects on traits expressed by their female partners (fecundity, egg size, longevity). These results demonstrate important consequences of sex-specific mitonuclear epistasis for both mating partners, consistent with a role for mitonuclear genetic constraints upon sex-specific adaptive evolution.
Figures


Similar articles
-
Complex mitonuclear interactions and metabolic costs of mating in male seed beetles.J Evol Biol. 2016 Feb;29(2):360-70. doi: 10.1111/jeb.12789. Epub 2015 Nov 28. J Evol Biol. 2016. PMID: 26548644
-
Sex-specific mitonuclear epistasis and the evolution of mitochondrial bioenergetics, ageing, and life history in seed beetles.Evolution. 2017 Feb;71(2):274-288. doi: 10.1111/evo.13109. Epub 2016 Nov 24. Evolution. 2017. PMID: 27861795
-
Mitochondrial-nuclear coadaptation revealed through mtDNA replacements in Saccharomyces cerevisiae.BMC Evol Biol. 2020 Sep 25;20(1):128. doi: 10.1186/s12862-020-01685-6. BMC Evol Biol. 2020. PMID: 32977769 Free PMC article.
-
The role of mitonuclear incompatibilities in allopatric speciation.Cell Mol Life Sci. 2022 Jan 29;79(2):103. doi: 10.1007/s00018-021-04059-3. Cell Mol Life Sci. 2022. PMID: 35091831 Free PMC article. Review.
-
Mitonuclear Mate Choice: A Missing Component of Sexual Selection Theory?Bioessays. 2018 Mar;40(3). doi: 10.1002/bies.201700191. Epub 2018 Feb 6. Bioessays. 2018. PMID: 29405334 Review.
Cited by
-
Sex-Specific Dominance of Gene Expression in Seed Beetles.Mol Biol Evol. 2024 Dec 6;41(12):msae244. doi: 10.1093/molbev/msae244. Mol Biol Evol. 2024. PMID: 39692633 Free PMC article.
-
Mitonuclear effects on sex ratio persist across generations in interpopulation hybrids.J Evol Biol. 2024 Nov 2;37(11):1386-1393. doi: 10.1093/jeb/voae123. J Evol Biol. 2024. PMID: 39324636
-
Mitonuclear genomics and aging.Hum Genet. 2020 Mar;139(3):381-399. doi: 10.1007/s00439-020-02119-5. Epub 2020 Jan 29. Hum Genet. 2020. PMID: 31997134 Free PMC article. Review.
-
Toward the Development of the Trojan Female Technique in Pest Insects: Male-Specific Influence of Mitochondrial Haplotype on Reproductive Output in the Seed Beetle Acanthoscelides obtectus.Evol Appl. 2024 Dec 26;17(12):e70065. doi: 10.1111/eva.70065. eCollection 2024 Dec. Evol Appl. 2024. PMID: 39726737 Free PMC article.
-
Mother's curse is pervasive across a large mitonuclear Drosophila panel.Evol Lett. 2021 Mar 13;5(3):230-239. doi: 10.1002/evl3.221. eCollection 2021 Jun. Evol Lett. 2021. PMID: 34136271 Free PMC article.
References
-
- Arnqvist G, Dowling DK, Eady P, Gay L, Tregenza T, Tuda M et al. (2010). Genetic architecture of metabolic rate: environment specific epistasis between mitochondrial and nuclear genes in an insect. Evolution 64: 3354–3363. - PubMed
-
- Arnqvist G, Rowe L. (2005) Sexual Conflict. Princeton University Press: New Jersey.
-
- Bates D, Maechler M, Bolker BM, Walker S (2014). lme4: Linear mixed-effects models using Eigen and S4. R package. 1.1-7 http://CRAN.R-project.org/package=lme4.
-
- Bonduriansky R, Chenoweth SF. (2009). Intralocus sexual conflict. Trends Ecol Evol 24: 280–288. - PubMed

