Delivery of ziconotide to cerebrospinal fluid via intranasal pathway for the treatment of chronic pain
- PMID: 26732557
- PMCID: PMC4747835
- DOI: 10.1016/j.jconrel.2015.12.044
Delivery of ziconotide to cerebrospinal fluid via intranasal pathway for the treatment of chronic pain
Abstract
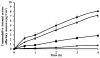
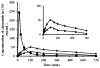
The purpose of the current study was to investigate the plausibility of delivery of ziconotide to the cerebrospinal fluid (CSF) via intranasal administration. Ziconotide was administered either in the form of solution or Kolliphor P 407 gels (KP 407) intranasally in Sprague-Dawley rats. The effect of incorporation of chitosan in the formulation was also investigated. Time course of drug in the CSF was investigated by collecting CSF from cisterna magna. Pharmacokinetics of ziconotide in CSF following intrathecal and intravenous (i.v.) administration of ziconotide was investigated. Upon intrathecal administration the elimination rate constant of ziconotide in CSF was found to be 1.01±0.34h(-1). The Cmax and Tmax of ziconotide in CSF following intravenous administration were found to be 37.78±6.8ng/mL and ~2h respectively. The time required to attain maximum concentration (Tmax) in CSF was less upon intranasal administration (15min) compared to i.v. administration (120min). Presence of chitosan enhanced the overall bioavailability of ziconotide from intranasal solution and gel formulations. The elimination rate constant of ziconotide in CSF following intranasal and intravenous administration of ziconotide solution was found to be 0.54±0.08h(-1) and 0.42±0.10h(-1) respectively. Whereas, intranasal administration of ziconotide in the form of in situ forming gel lowered the elimination rate significantly. These results suggest that intranasal administration could be a potential noninvasive and patient compliant method of delivering ziconotide to CSF to treat chronic pain.
Keywords: Cerebrospinal fluid; In situ gels; Intranasal; Ziconotide.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures






References
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European journal of pain. 2006;10:287–287. - PubMed
-
- Scholz J, Woolf CJ. Can we conquer pain? Nature neuroscience. 2002;5:1062–1067. - PubMed
-
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, Fisher R, Bryce DA, Mangieri EA, Luther RR. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. Jama. 2004;291:63–70. - PubMed
-
- Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug discovery today. 2010;15:40–56. - PubMed
-
- Diao L, Meibohm B. Pharmacokinetics and pharmacokinetic–pharmacodynamic correlations of therapeutic peptides. Clinical pharmacokinetics. 2013;52:855–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

