Pharmacokinetics of mefloquine and its effect on sulfamethoxazole and trimethoprim steady-state blood levels in intermittent preventive treatment (IPTp) of pregnant HIV-infected women in Kenya
- PMID: 26732683
- PMCID: PMC4700759
- DOI: 10.1186/s12936-015-1049-9
Pharmacokinetics of mefloquine and its effect on sulfamethoxazole and trimethoprim steady-state blood levels in intermittent preventive treatment (IPTp) of pregnant HIV-infected women in Kenya
Abstract
Background: Intermittent preventive treatment in pregnancy with sulfadoxine/pyrimethamine is contra-indicated in HIV-positive pregnant women receiving sulfamethoxazole/trimethoprim prophylaxis. Since mefloquine is being considered as a replacement for sulfadoxine/pyrimethamine in this vulnerable population, an investigation on the pharmacokinetic interactions of mefloquine, sulfamethoxazole and trimethoprim in pregnant, HIV-infected women was performed.
Methods: A double-blinded, placebo-controlled study was conducted with 124 HIV-infected, pregnant women on a standard regimen of sulfamethoxazole/trimethoprim prophylaxis. Seventy-two subjects received three doses of mefloquine (15 mg/kg) at monthly intervals. Dried blood spots were collected from both placebo and mefloquine arms four to 672 h post-administration and on day 7 following a second monthly dose of mefloquine. A novel high-performance liquid chromatographic method was developed to simultaneously measure mefloquine, sulfamethoxazole and trimethoprim from each blood spot. Non-compartmental methods using a naïve-pooled data approach were used to determine mefloquine pharmacokinetic parameters.
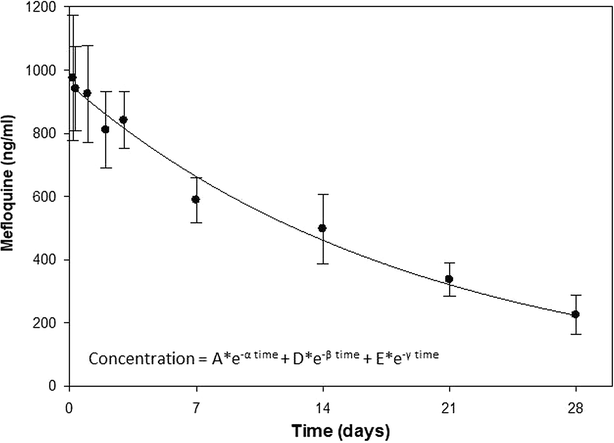
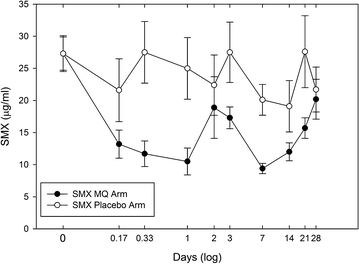
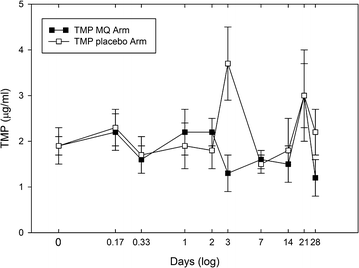
Results: Sulfamethoxazole/trimethoprim prophylaxis did not noticeably influence mefloquine pharmacokinetics relative to reported values. The mefloquine half-life, observed clearance (CL/f), and area-under-the-curve (AUC0→∞) were 12.0 days, 0.035 l/h/kg and 431 µg-h/ml, respectively. Although trimethoprim steady-state levels were not significantly different between arms, sulfamethoxazole levels showed a significant 53% decrease after mefloquine administration relative to the placebo group and returning to pre-dose levels at 28 days.
Conclusions: Although a transient decrease in sulfamethoxazole levels was observed, there was no change in hospital admissions due to secondary bacterial infections, implying that mefloquine may have provided antimicrobial protection.
Figures
Similar articles
-
Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-infected women receiving cotrimoxazole prophylaxis: a multicenter randomized placebo-controlled trial.PLoS Med. 2014 Sep 23;11(9):e1001735. doi: 10.1371/journal.pmed.1001735. eCollection 2014 Sep. PLoS Med. 2014. PMID: 25247995 Free PMC article. Clinical Trial.
-
Safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in pregnant women with HIV from Gabon and Mozambique: a randomised, double-blind, placebo-controlled trial.Lancet Infect Dis. 2024 May;24(5):476-487. doi: 10.1016/S1473-3099(23)00738-7. Epub 2024 Jan 12. Lancet Infect Dis. 2024. PMID: 38224706 Clinical Trial.
-
Insights Into Circulating Cytokine Dynamics During Pregnancy in HIV-Infected Beninese Exposed to Plasmodium falciparum Malaria.Am J Trop Med Hyg. 2015 Aug;93(2):287-92. doi: 10.4269/ajtmh.14-0653. Epub 2015 Jun 22. Am J Trop Med Hyg. 2015. PMID: 26101276 Free PMC article. Clinical Trial.
-
Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa.Lancet Infect Dis. 2006 Feb;6(2):100-11. doi: 10.1016/S1473-3099(06)70383-8. Lancet Infect Dis. 2006. PMID: 16439330 Review.
-
Intermittent preventive treatment of malaria in pregnancy: at the crossroads of public health policy.Trop Med Int Health. 2011 Jul;16(7):774-85. doi: 10.1111/j.1365-3156.2011.02765.x. Epub 2011 Apr 7. Trop Med Int Health. 2011. PMID: 21477099 Review.
Cited by
-
Population Pharmacokinetics and Pharmacodynamics of Lumefantrine in Young Ugandan Children Treated With Artemether-Lumefantrine for Uncomplicated Malaria.J Infect Dis. 2016 Oct 15;214(8):1243-51. doi: 10.1093/infdis/jiw338. Epub 2016 Jul 28. J Infect Dis. 2016. PMID: 27471317 Free PMC article. Clinical Trial.
-
Clinical needs assessment to inform development of a new assay to detect antimalarial drugs in patient samples: A case study.PLOS Glob Public Health. 2023 Aug 24;3(8):e0002087. doi: 10.1371/journal.pgph.0002087. eCollection 2023. PLOS Glob Public Health. 2023. PMID: 37616192 Free PMC article.
References
-
- Ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, Van Eijk AM, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71(suppl 2):41–54. - PubMed
-
- WHO. Malaria in HIV/Aids patients. Geneva: World Health Organization. http://who.int/malaria/areas/high_risk_groups/hiv_aids_patients/en/index....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical