When stem cells grow old: phenotypes and mechanisms of stem cell aging
- PMID: 26732838
- PMCID: PMC4725211
- DOI: 10.1242/dev.130633
When stem cells grow old: phenotypes and mechanisms of stem cell aging
Abstract
All multicellular organisms undergo a decline in tissue and organ function as they age. An attractive theory is that a loss in stem cell number and/or activity over time causes this decline. In accordance with this theory, aging phenotypes have been described for stem cells of multiple tissues, including those of the hematopoietic system, intestine, muscle, brain, skin and germline. Here, we discuss recent advances in our understanding of why adult stem cells age and how this aging impacts diseases and lifespan. With this increased understanding, it is feasible to design and test interventions that delay stem cell aging and improve both health and lifespan.
Keywords: Age-related diseases; Hematopoietic stem cells; Multicellular organisms.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
D.A.S. is a consultant to and inventor on patents licensed to Ovascience.
Figures
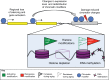

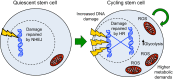
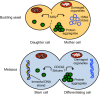
References
-
- Ahlenius H., Visan V., Kokaia M., Lindvall O. and Kokaia Z. (2009). Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J. Neurosci. 29, 4408-4419. 10.1523/JNEUROSCI.6003-08.2009 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

