Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study)
- PMID: 26733110
- PMCID: PMC5099201
- DOI: 10.1136/annrheumdis-2015-208426
Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study)
Abstract
Objective: To compare the efficacy and safety between tocilizumab added to methotrexate and tocilizumab switched from methotrexate in patients with active rheumatoid arthritis (RA).
Methods: This is a 2-year randomised, controlled study. RA patients with moderate or high disease activity despite methotrexate were randomly assigned either to tocilizumab added to methotrexate (add-on) or tocilizumab switched from methotrexate (switch). The primary endpoint was the DAS28 remission rate at week 24. Secondary objectives included other clinical efficacy indices, radiological outcomes assessed with the van der Heijde-modified total Sharp scoring system (mTSS), and safety.
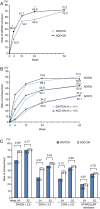
Results: Of 223 randomised patients, 83% completed 52 weeks. DAS28 remission rates at week 24 were 70% for add-on and 55% for switch (p=0.02), but they became comparable at week 52 (72% vs 70%, p=0.86). Structural remission rates (mTSS≤0.5) at week 52 were not different (66% vs 64%, p=0.92). However, clinically relevant radiographic progression rates (CRRP; mTSS≥3) tended to be higher with the switch than with the add-on (15% vs 7%, p=0.07). Radiographic progression in the CRRP patients was larger with the switch than with the add-on (9.0/year vs 5.0/year, p=0.04). The difference in the mean C-reactive protein of the CRRP patients was significant for the first 24 weeks (1.56 vs 0.49, p=0.001) but not for the following 28 weeks (0.10 vs 0.04, p=0.1). Overall safety was preferable in the switch group.
Conclusions: In RA patients with inadequate response to methotrexate, tocilizumab added to methotrexate more rapidly suppressed inflammation than tocilizumab switched from methotrexate, leading to superior clinical efficacy and prevention of joint destruction.
Trial registration number: NCT01120366.
Keywords: DMARDs (biologic); Disease Activity; Rheumatoid Arthritis; Treatment.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
YK has received lecture fees from AbbVie GK, Eisai Co, Chugai Pharmaceutical Co, Eli Lilly Japan K.K., Mitsubishi-Tanabe, Bristol–Myers K.K., Astellas Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Pfizer, Janssen, Santen, Kyowa Hakko Kirin and UCB Japan. TA has received research grants or lecture fees from Eisai Co, Chugai Pharmaceutical Co, Novartis, Mitsubishi-Tanabe, Teijin, Astellas, Takeda, Novo Nordisk, Abbvie GK, Pfizer and Bristol–Myers K.K. YT has received consulting fees, speaking fees and/or honoraria from Abbvie GK, Daiichi-Sankyo, Chugai Pharmaceutical Co, Takeda, Mitsubishi-Tanabe, Bristol–Myers K.K., Astellas, Eisai Co, Janssen, Pfizer, Asahikasei Pharma Corp., Eli Lilly Japan K.K., GlaxoSmithKline, UCB Japan, Teijin, MSD and Santen and has received research grants from Mitsubishi-Tanabe, Takeda, Chugai Pharmaceutical Co, Astellas, Eisa Co.i, Taisho-Toyama, Kyowa-Kirin, AbbVie GK and Bristol–Myers K.K. KA has received research grants from Chugai Pharmaceutical Co. and Astellas, and has received lecture fees from Abbvie GK, Astellas, Bristol–Myers K.K., Chugai Pharmaceutical Co and Pfizer. SH has received lecture fees from AbbVie GK, Eisai Co an Bristol–Myers K.K. YM has received research grants or lecture fees from Japan Blood Products, Taisho Toyama, Nippon Kayaku, Takeda Pharma, AbbVie GK, Janssen Pharma. and UCB Japan, Astellas, Chugai Pharmaceutical Co, Ono, Mitsubishi-Tanabe, Teijin and Eisai Co. SH has received lecture fees from AbbVie GK, Eisai and Bristol-Myers K.K. ET has received lecture fees or consulting fees from Abbvie GK, Eisai Co, Chugai Pharmaceutical Co, Bristol–Myers K.K., Astellas Pharmaceutical, Pfizer, Takeda Pharmaceutical and Santen Pharmaceutical. HY has received research grants or lecture fees from AbbVie GK, Bristol-Myers K.K., Chugai Pharmaceutical Co, Eisai Co, Mitsubishi-Tanabe, Astellas, Pfizer, Takeda, UCB Japan and Nippon Shinyaku. KY has received consultancy fees from Abbott, Bristol-Myers K.K., Chugai Pharmaceutical Co, Eisai Co, Mitsubishi-Tanabe, Pfizer, Roche and UCB Pharma, and has received research grants from AbbVie GK, Eisai Co, Mitsubishi-Tanabe, Pfizer, Santen and UCB Japan. TT has received research grants or lecture fees from Astellas Pharma, Bristol-Myers K.K., Chugai Pharmaceutical Co, Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Co., Pfizer Japan Inc., Santen Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., AbbVie GK, Asahikasei Pharma Corp., Taisho Toyama Pharmaceutical Co., Ltd., SymBio Pharmaceuticals Ltd., Janssen Pharmaceutical K.K., Celtrion, Nipponkayaku Co. Ltd. and UCB Japan, and consultant fees from Astra Zeneca K.K., Eli Lilly Japan K.K., Novartis Pharma K.K., Mitsubishi Tanabe Pharma Co., Asahi Kasei Medical K.K., Abbvie GK, Daiichi Sankyo Co., Ltd., Bristol–Myers K.K. and Nipponkayaku Co. Ltd.
Figures


References
-
- Klareskog L, van der Heijde D, de Jager JP, et al. , TEMPO (Trial of Etanercept and Methotrexate with Radiographic Patient Outcomes) study investigators. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. 10.1016/S0140-6736(04)15640-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

