MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2
- PMID: 26733151
- PMCID: PMC4702409
- DOI: 10.1186/s13045-015-0229-y
MicroRNA-26a suppresses epithelial-mesenchymal transition in human hepatocellular carcinoma by repressing enhancer of zeste homolog 2
Abstract
Background: Our previous study reported that microRNA-26a (miR-26a) inhibited tumor progression by inhibiting tumor angiogenesis and intratumoral macrophage infiltration in hepatocellular carcinoma (HCC). The direct roles of miR-26a on tumor cell invasion remain poorly understood. In this study, we aim to explore the mechanism of miR-26a in modulating epithelial-mesenchymal transition (EMT) in HCC.
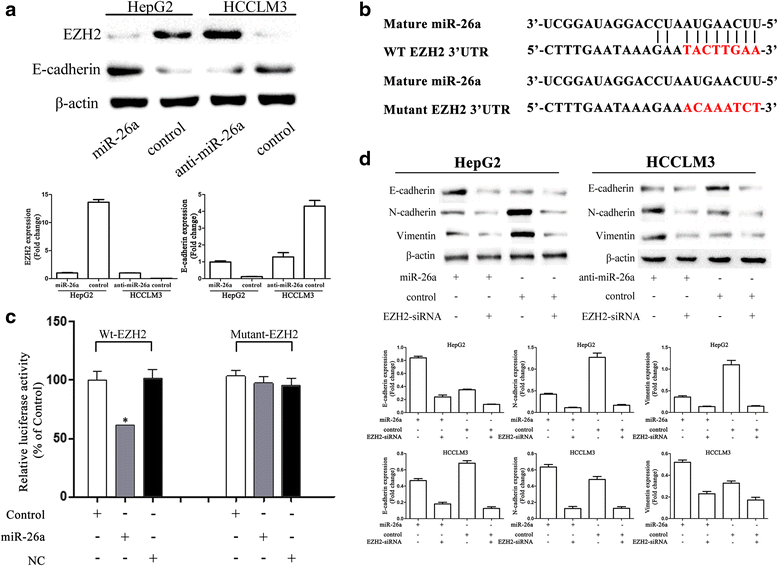
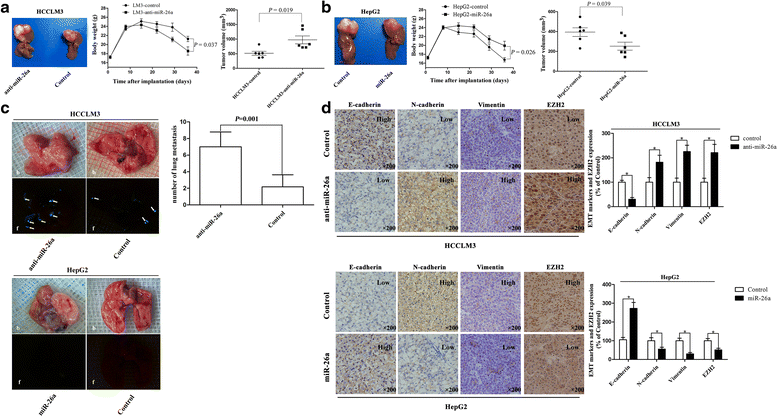
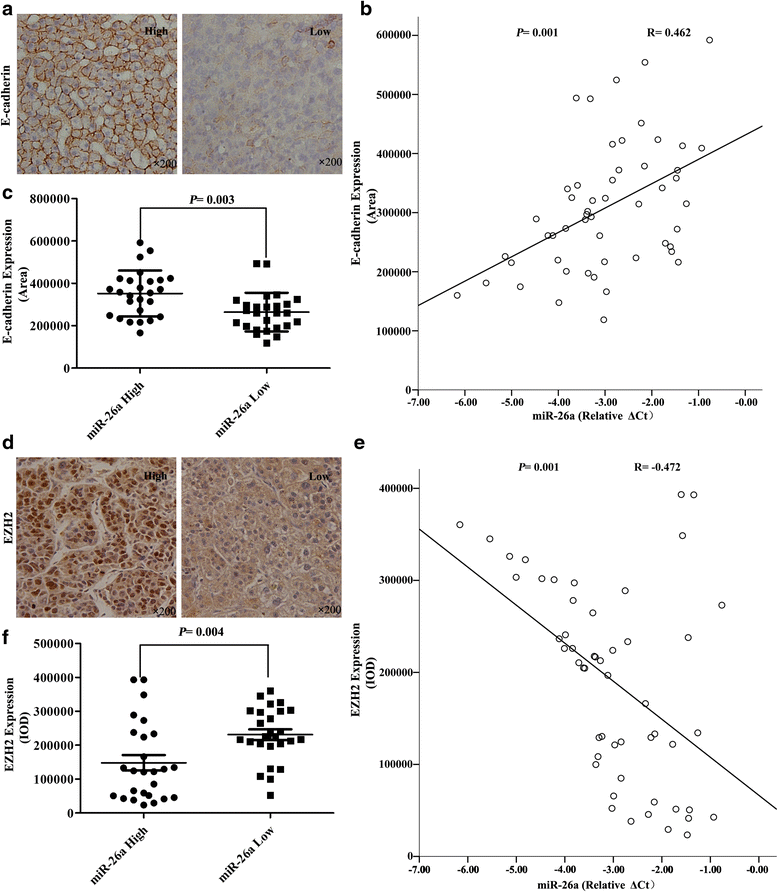
Methods: In vitro cell morphology and cell migration were compared between the hepatoma cell lines HCCLM3 and HepG2, which were established in the previous study. Overexpression and down-regulation of miR-26a were induced in these cell lines, and Western blot and immunofluorescence assays were used to detect the expression of EMT markers. Xenograft nude mouse models were used to observe tumor growth and pulmonary metastasis. Immunohistochemical assays were conducted to study the relationships between miR-26a expression and enhancer of zeste homolog 2 (EZH2) and E-cadherin expression in human HCC samples.
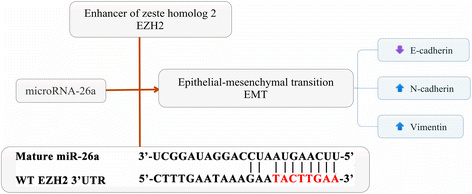
Results: Down-regulation of miR-26a in HCCLM3 and HepG2 cells resulted in an EMT-like cell morphology and high motility in vitro and increased in tumor growth and pulmonary metastasis in vivo. Through down-regulation of EZH2 expression and up-regulation of E-cadherin expression, miR-26a inhibited the EMT process in vitro and in vivo. Luciferase reporter assay showed that miR-26a directly interacted with EZH2 messenger RNA (mRNA). Furthermore, the expression of miR-26a was positively correlated with E-cadherin expression and inversely correlated with EZH2 expression in human HCC tissue.
Conclusions: miR-26a inhibited the EMT process in HCC by down-regulating EZH2 expression.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

