HIV-1 Nef-associated Factor 1 Enhances Viral Production by Interacting with CRM1 to Promote Nuclear Export of Unspliced HIV-1 gag mRNA
- PMID: 26733199
- PMCID: PMC4813482
- DOI: 10.1074/jbc.M115.706135
HIV-1 Nef-associated Factor 1 Enhances Viral Production by Interacting with CRM1 to Promote Nuclear Export of Unspliced HIV-1 gag mRNA
Abstract
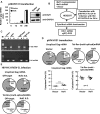
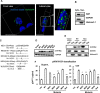
HIV-1 depends on host-cell-encoded factors to complete its life cycle. A comprehensive understanding of how HIV-1 manipulates host machineries during viral infection can facilitate the identification of host targets for antiviral drugs or gene therapy. The cellular protein Naf1 (HIV-1 Nef-associated factor 1) is a CRM1-dependent nucleo-cytoplasmic shuttling protein, and has been identified to regulate multiple receptor-mediated signal pathways in inflammation. The cytoplasm-located Naf1 can inhibit NF-κB activation through binding to A20, and the loss of Naf1 controlled NF-κB activation is associated with multiple autoimmune diseases. However, the effect of Naf1 on HIV-1 mRNA expression has not been characterized. In this study we found that the nucleus-located Naf1 could promote nuclear export of unspliced HIV-1 gag mRNA. We demonstrated that the association between Naf1 and CRM1 was required for this function as the inhibition or knockdown of CRM1 expression significantly impaired Naf1-promoted HIV-1 production. The mutation of Naf1 nuclear export signals (NESs) that account for CRM1 recruitment for nuclear export decreased Naf1 function. Additionally, the mutation of the nuclear localization signal (NLS) of Naf1 diminished its ability to promote HIV-1 production, demonstrating that the shuttling property of Naf1 is required for this function. Our results reveal a novel role of Naf1 in enhancing HIV-1 production, and provide a potential therapeutic target for controlling HIV-1 infection.
Keywords: Cellular factor; Chromosome region maintenance 1; HIV-1; Nef-associated factor 1; Nuclear export; human immunodeficiency virus (HIV); microbiology; molecular cell biology; virology; virus.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
ANP32A and ANP32B are key factors in the Rev-dependent CRM1 pathway for nuclear export of HIV-1 unspliced mRNA.J Biol Chem. 2019 Oct 18;294(42):15346-15357. doi: 10.1074/jbc.RA119.008450. Epub 2019 Aug 23. J Biol Chem. 2019. PMID: 31444273 Free PMC article.
-
Naf1 Regulates HIV-1 Latency by Suppressing Viral Promoter-Driven Gene Expression in Primary CD4+ T Cells.J Virol. 2016 Dec 16;91(1):e01830-16. doi: 10.1128/JVI.01830-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795436 Free PMC article.
-
The export receptor Crm1 forms a dimer to promote nuclear export of HIV RNA.Elife. 2014 Dec 8;3:e04121. doi: 10.7554/eLife.04121. Elife. 2014. PMID: 25486595 Free PMC article.
-
Atomic basis of CRM1-cargo recognition, release and inhibition.Semin Cancer Biol. 2014 Aug;27:52-61. doi: 10.1016/j.semcancer.2014.03.002. Epub 2014 Mar 12. Semin Cancer Biol. 2014. PMID: 24631835 Free PMC article. Review.
-
HIV-1 Rev multimerization: mechanism and insights.Curr HIV Res. 2013 Dec;11(8):623-34. doi: 10.2174/1570162x12666140307094603. Curr HIV Res. 2013. PMID: 24606219 Review.
Cited by
-
ANP32A and ANP32B are key factors in the Rev-dependent CRM1 pathway for nuclear export of HIV-1 unspliced mRNA.J Biol Chem. 2019 Oct 18;294(42):15346-15357. doi: 10.1074/jbc.RA119.008450. Epub 2019 Aug 23. J Biol Chem. 2019. PMID: 31444273 Free PMC article.
-
Immune regulator ABIN1 suppresses HIV-1 transcription by negatively regulating the ubiquitination of Tat.Retrovirology. 2017 Feb 13;14(1):12. doi: 10.1186/s12977-017-0338-5. Retrovirology. 2017. PMID: 28193275 Free PMC article.
-
Host factor Naf1 restricts HIV-1 infection of myeloid cells and compromises the capacity of dendritic cell to prime CD4+ T cell.Virol Sin. 2025 Apr;40(2):217-224. doi: 10.1016/j.virs.2025.03.007. Epub 2025 Mar 24. Virol Sin. 2025. PMID: 40139499 Free PMC article.
-
Scaffold attachment factor B suppresses HIV-1 infection of CD4+ T cells by preventing binding of RNA polymerase II to HIV-1's long terminal repeat.J Biol Chem. 2018 Aug 3;293(31):12177-12185. doi: 10.1074/jbc.RA118.002018. Epub 2018 Jun 10. J Biol Chem. 2018. PMID: 29887524 Free PMC article.
-
From Entry to Egress: Strategic Exploitation of the Cellular Processes by HIV-1.Front Microbiol. 2020 Dec 4;11:559792. doi: 10.3389/fmicb.2020.559792. eCollection 2020. Front Microbiol. 2020. PMID: 33343516 Free PMC article. Review.
References
-
- Brass A. L., Dykxhoorn D. M., Benita Y., Yan N., Engelman A., Xavier R. J., Lieberman J., and Elledge S. J. (2008) Identification of Host Proteins Required for HIV Infection Through a Functional Genomic Screen. Science 319, 921–926 - PubMed
-
- König R., Zhou Y., Elleder D., Diamond T. L., Bonamy G. M. C., Irelan J. T., Chiang C.-y., Tu B. P., De Jesus P. D., Lilley C. E., Seidel S., Opaluch A. M., Caldwell J. S., Weitzman M. D., Kuhen K. L., Bandyopadhyay S., Ideker T., Orth A. P., Miraglia L. J., Bushman F. D., Young J. A., and Chanda S. K. (2008) Global Analysis of Host-Pathogen Interactions that Regulate Early-Stage HIV-1 Replication. Cell 135, 49–60 - PMC - PubMed
-
- Zhou H., Xu M., Huang Q., Gates A. T., Zhang X. D., Castle J. C., Stec E., Ferrer M., Strulovici B., Hazuda D. J., and Espeseth A. S. (2008) Genome-Scale RNAi Screen for Host Factors Required for HIV Replication. Cell Host Microbe 4, 495–504 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

