Overexpression of Swedish mutant APP in aged astrocytes attenuates excitatory synaptic transmission
- PMID: 26733247
- PMCID: PMC4760399
- DOI: 10.14814/phy2.12665
Overexpression of Swedish mutant APP in aged astrocytes attenuates excitatory synaptic transmission
Abstract
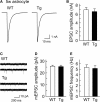
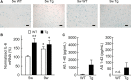
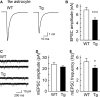
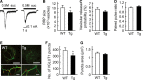
Amyloid precursor protein (APP), a type I transmembrane protein, has different aspects, namely, performs essential physiological functions and produces β-amyloid peptide (Aβ). Overexpression of neuronal APP is responsible for synaptic dysfunction. In the central nervous system, astrocytes - a major glial cell type - have an important role in the regulation of synaptic transmission. Although APP is expressed in astrocytes, it remains unclear whether astrocytic overexpression of mutant APP affects synaptic transmission. In this study, the effect of astrocytic overexpression of a mutant APP on the excitatory synaptic transmission was investigated using coculture system of the transgenic (Tg) cortical astrocytes that express the human APP695 polypeptide with the double mutation K670N + M671L found in a large Swedish family with early onset Alzheimer's disease, and wild-type hippocampal neuron. Significant secretion of Aβ 1-40 and 1-42 was observed in cultured cortical astrocytes from the Tg2576 transgenic mouse that genetically overexpresses Swedish mutant APP. Under the condition, Tg astrocytes did not affect excitatory synaptic transmission of cocultured wild-type neurons. However, aged Tg astrocytes cultured for 9 weeks elicited a significant decrease in excitatory synaptic transmission in cocultured neurons. Moreover, a reduction in the number of readily releasable synaptic vesicles accompanied a decrease in the number of excitatory synapses in neurons cocultured with aged Tg astrocytes. These observations indicate that astrocytic expression of the mutant APP is involved in the downregulation of synaptic transmission with age.
Keywords: Aging; alzheimer's disease; amyloid; astrocyte; synaptic release.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

Similar articles
-
Long-term culture of astrocytes attenuates the readily releasable pool of synaptic vesicles.PLoS One. 2012;7(10):e48034. doi: 10.1371/journal.pone.0048034. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110166 Free PMC article.
-
Astrocytes with previous chronic exposure to amyloid β-peptide fragment 1-40 suppress excitatory synaptic transmission.J Neurochem. 2017 Dec;143(6):624-634. doi: 10.1111/jnc.14247. Epub 2017 Nov 10. J Neurochem. 2017. PMID: 29076533
-
Age-related impairment of synaptic transmission but normal long-term potentiation in transgenic mice that overexpress the human APP695SWE mutant form of amyloid precursor protein.J Neurosci. 2001 Jul 1;21(13):4691-8. doi: 10.1523/JNEUROSCI.21-13-04691.2001. J Neurosci. 2001. PMID: 11425896 Free PMC article.
-
From synaptic spines to nuclear signaling: nuclear and synaptic actions of the amyloid precursor protein.J Neurochem. 2013 Jul;126(2):183-90. doi: 10.1111/jnc.12239. Epub 2013 Apr 3. J Neurochem. 2013. PMID: 23495999 Review.
-
Keeping Excitation-Inhibition Ratio in Balance.Int J Mol Sci. 2022 May 20;23(10):5746. doi: 10.3390/ijms23105746. Int J Mol Sci. 2022. PMID: 35628556 Free PMC article. Review.
Cited by
-
α-Mangostin decreases β-amyloid peptides production via modulation of amyloidogenic pathway.CNS Neurosci Ther. 2017 Jun;23(6):526-534. doi: 10.1111/cns.12699. Epub 2017 Apr 21. CNS Neurosci Ther. 2017. PMID: 28429536 Free PMC article.
-
The role of astrocytes in amyloid production and Alzheimer's disease.Open Biol. 2017 Dec;7(12):170228. doi: 10.1098/rsob.170228. Open Biol. 2017. PMID: 29237809 Free PMC article. Review.
-
Quantitative Analysis of Presynaptic Vesicle Luminal pH in Cultured Neurons.Methods Mol Biol. 2022;2417:45-58. doi: 10.1007/978-1-0716-1916-2_4. Methods Mol Biol. 2022. PMID: 35099790
-
Rapid Identification of 3,6'-Disinapoyl Sucrose Metabolites in Alzheimer's Disease Model Mice Using UHPLC-Orbitrap Mass Spectrometry.Molecules. 2021 Dec 25;27(1):114. doi: 10.3390/molecules27010114. Molecules. 2021. PMID: 35011346 Free PMC article.
-
Defined astrocytic expression of human amyloid precursor protein in Tg2576 mouse brain.Glia. 2019 Feb;67(2):393-403. doi: 10.1002/glia.23550. Epub 2018 Nov 28. Glia. 2019. PMID: 30485540 Free PMC article.
References
-
- Alarcon, R. , Fuenzalida C., Santibanez M., and Von Bernhardi R.. 2005. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface‐bound β‐amyloid. J. Biol. Chem. 280:30406–30415. - PubMed
-
- Allen, N. J. , and Barres B. A.. 2009. Neuroscience: glia ‐ more than just brain glue. Nature 457:675–677. - PubMed
-
- Bekkers, J. M. , and Stevens C. F.. 1995. Quantal analysis of EPSCs recorded from small numbers of synapses in hippocampal cultures. J. Neurophysiol. 73:1145–1156. - PubMed
-
- Berkenbosch, F. , Refolo L. M., V. L., Friedrich, Jr , Casper D., Blum M., and Robakis N. K.. 1990. The Alzheimer's amyloid precursor protein is produced by type I astrocytes in primary cultures of rat neuroglia. J. Neurosci. Res., 25:431–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

