Role of HDACs in optic nerve damage-induced nuclear atrophy of retinal ganglion cells
- PMID: 26733303
- PMCID: PMC5125391
- DOI: 10.1016/j.neulet.2015.12.012
Role of HDACs in optic nerve damage-induced nuclear atrophy of retinal ganglion cells
Abstract
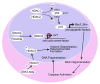
Optic neuropathies are characterized by retinal ganglion cell (RGC) death, resulting in the loss of vision. In glaucoma, the most common optic neuropathy, RGC death is initiated by axonal damage, and can be modeled by inducing acute axonal trauma through procedures such as optic nerve crush (ONC) or optic nerve axotomy. One of the early events of RGC death is nuclear atrophy, and is comprised of RGC-specific gene silencing, histone deacetylation, heterochromatin formation, and nuclear shrinkage. These early events appear to be principally regulated by epigenetic mechanisms involving histone deacetylation. Class I histone deacetylases HDACs 1, 2, and 3 are known to play important roles in the process of early nuclear atrophy in RGCs, and studies using both inhibitors and genetic ablation of Hdacs also reveal a critical role in the cell death process. Select inhibitors, such as those being developed for cancer therapy, may also provide a viable secondary treatment option for optic neuropathies.
Keywords: Apoptosis; Glaucoma; Heterochromatin; Histone deacetylase (HDAC); Neurodegeneration; Nuclear atrophy; Optic nerve injury; Retinal ganglion cell.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

