Optimizing Social Network Support to Families Living With Parental Cancer: Research Protocol for the Cancer-PEPSONE Study
- PMID: 26733339
- PMCID: PMC4712344
- DOI: 10.2196/resprot.5055
Optimizing Social Network Support to Families Living With Parental Cancer: Research Protocol for the Cancer-PEPSONE Study
Abstract
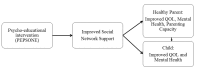
Background: Parental cancer can have a significant impact on a family's psychosocial functioning and quality of life, whereby the children's situation is strongly related to parental coping and capacity. Such parents ask for more help in order to increase their care capacity, while the network is often insecure about how to help and thereby withdraw. They ask for guidance and training to be able to support cancer families. Based on this, the Cancer- Psycho-Educational Program for the SOcial NEtwork (PEPSONE) study was developed.
Objective: To optimize social network support through a psycho-educational program for families living with parental cancer and their network members in order to increase parental capacity and thereby secure the children's safety and quality of life.
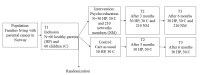
Methods: A randomized controlled trial (RCT) in which families (N=60) living with parental cancer will be randomized to either an intervention group or a control group. The intervention will last for 3 hours and includes (1) introduction, (2) psycho-education (living with cancer in the family and the importance of social network support), and (3) discussion (this family's need for social support). Primary outcomes are social support, mental health, and quality of life, and secondary outcomes are resilience and parental capacity. Data will be collected by a set of questionnaires distributed to healthy parents (N=60) living with a partner with cancer, one child in the family between 8-18 years of age (N=60), and network members (N=210) of the intervention families at inclusion, and after 3 and 6 months. Comparing differences between the intervention group (n=30) and the control group (n=30), the power analysis shows that P<.05 and a statistical power = .80 would detect effect sizes of clinical interest.
Results: This paper presents the Cancer-PEPSON study's protocol to provide a broader understanding of the background and content of the program. The study is ongoing until August 2016 and the first results are anticipated to be finished by November 2015.
Conclusions: To our knowledge, this will be the first RCT study to optimize social network support through a psycho-educational program for families living with parental cancer and their network members, as well as provide an evidence basis for social network support. The results may provide important knowledge that is useful for clinical practice and further research. The trial is reported according to the CONSORT checklist.
Clinicaltrial: International Standard Randomized Controlled Trial Number (ISRCTN): 15982171; http://www.controlled-trials.com/ISRCTN15982171/15982171 (Archived by WebCite at http://www.webcitation.org/6cg9zunS0).
Keywords: children; mental health; parental cancer; psycho-education; quality of life; randomized controlled trial; social support.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Cancer Registry of Norway . Cancer in Norway 2013 - Cancer incidence, mortality, survival and prevalence in Norway. Oslo, Norway: 2014. [2015-11-02]. http://www.kreftregisteret.no/
-
- Niemelä M, Paananen R, Hakko H, Merikukka M, Gissler M, Räsänen S. The prevalence of children affected by parental cancer and their use of specialized psychiatric services: the 1987 Finnish Birth Cohort study. Int J Cancer. 2012 Nov 1;131(9):2117–25. doi: 10.1002/ijc.27466. doi: 10.1002/ijc.27466. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources