RPS23RG1 reduces Aβ oligomer-induced synaptic and cognitive deficits
- PMID: 26733416
- PMCID: PMC4702092
- DOI: 10.1038/srep18668
RPS23RG1 reduces Aβ oligomer-induced synaptic and cognitive deficits
Abstract
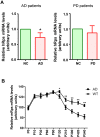
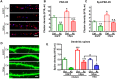
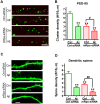
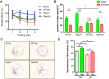
Alzheimer's disease (AD) is the most common form of dementia in the elderly. It is generally believed that β-amyloidogenesis, tau-hyperphosphorylation, and synaptic loss underlie cognitive decline in AD. Rps23rg1, a functional retroposed mouse gene, has been shown to reduce Alzheimer's β-amyloid (Aβ) production and tau phosphorylation. In this study, we have identified its human homolog, and demonstrated that RPS23RG1 regulates synaptic plasticity, thus counteracting Aβ oligomer (oAβ)-induced cognitive deficits in mice. The level of RPS23RG1 mRNA is significantly lower in the brains of AD compared to non-AD patients, suggesting its potential role in the pathogenesis of the disease. Similar to its mouse counterpart, human RPS23RG1 interacts with adenylate cyclase, activating PKA/CREB, and inhibiting GSK-3. Furthermore, we show that human RPS23RG1 promotes synaptic plasticity and offsets oAβ-induced synaptic loss in a PKA-dependent manner in cultured primary neurons. Overexpression of Rps23rg1 in transgenic mice consistently prevented oAβ-induced PKA inactivation, synaptic deficits, suppression of long-term potentiation, and cognitive impairment as compared to wild type littermates. Our study demonstrates that RPS23RG1 may reduce the occurrence of key elements of AD pathology and enhance synaptic functions to counteract oAβ-induced synaptic and cognitive deficits in AD.
Figures


Similar articles
-
The Rps23rg gene family originated through retroposition of the ribosomal protein s23 mRNA and encodes proteins that decrease Alzheimer's beta-amyloid level and tau phosphorylation.Hum Mol Genet. 2010 Oct 1;19(19):3835-43. doi: 10.1093/hmg/ddq302. Epub 2010 Jul 22. Hum Mol Genet. 2010. PMID: 20650958 Free PMC article.
-
RPS23RG1 Is Required for Synaptic Integrity and Rescues Alzheimer's Disease-Associated Cognitive Deficits.Biol Psychiatry. 2019 Aug 1;86(3):171-184. doi: 10.1016/j.biopsych.2018.08.009. Epub 2018 Aug 25. Biol Psychiatry. 2019. PMID: 30292394 Free PMC article.
-
A functional mouse retroposed gene Rps23r1 reduces Alzheimer's beta-amyloid levels and tau phosphorylation.Neuron. 2009 Nov 12;64(3):328-40. doi: 10.1016/j.neuron.2009.08.036. Neuron. 2009. PMID: 19914182 Free PMC article.
-
Is interaction of amyloid β-peptides with metals involved in cognitive activity?Metallomics. 2015 Aug;7(8):1205-12. doi: 10.1039/c5mt00076a. Metallomics. 2015. PMID: 25959547 Review.
-
Synaptic Alterations in Mouse Models for Alzheimer Disease-A Special Focus on N-Truncated Abeta 4-42.Molecules. 2018 Mar 21;23(4):718. doi: 10.3390/molecules23040718. Molecules. 2018. PMID: 29561816 Free PMC article. Review.
Cited by
-
SORLA attenuates EphA4 signaling and amyloid β-induced neurodegeneration.J Exp Med. 2017 Dec 4;214(12):3669-3685. doi: 10.1084/jem.20171413. Epub 2017 Nov 7. J Exp Med. 2017. PMID: 29114064 Free PMC article.
-
Sanshen San Formula Hinders Cognitive Function and Pathology in Alzheimer's Disease Through Potentiating the Function of Synapse.CNS Neurosci Ther. 2025 Apr;31(4):e70349. doi: 10.1111/cns.70349. CNS Neurosci Ther. 2025. PMID: 40202070 Free PMC article.
-
Phenazopyridine promotes RPS23RG1/Rps23rg1 transcription and ameliorates Alzheimer-associated phenotypes in mice.Neuropsychopharmacology. 2022 Nov;47(12):2042-2050. doi: 10.1038/s41386-022-01373-7. Epub 2022 Jul 11. Neuropsychopharmacology. 2022. PMID: 35821069 Free PMC article.
-
ER-associated degradation regulates Alzheimer's amyloid pathology and memory function by modulating γ-secretase activity.Nat Commun. 2017 Nov 13;8(1):1472. doi: 10.1038/s41467-017-01799-4. Nat Commun. 2017. PMID: 29133892 Free PMC article.
-
Comment on "Activation of the Omega-3 Fatty Acid Receptor GPR120 Protects against Focal Cerebral Ischemic Injury by Preventing Inflammation and Apoptosis in Mice".J Immunol. 2022 Oct 1;209(7):1229-1233. doi: 10.4049/jimmunol.2200151. Epub 2022 Sep 20. J Immunol. 2022. PMID: 36167357 Free PMC article. No abstract available.
References
-
- Terry R. D. et al. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol 30, 572–580 (1991). - PubMed
-
- Zemlan F. P. et al. Quantification of axonal damage in traumatic brain injury: affinity purification and characterization of cerebrospinal fluid tau proteins. J Neurochem 72, 741–50 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

