Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads
- PMID: 26733606
- PMCID: PMC4729615
- DOI: 10.1161/CIRCULATIONAHA.115.018535
Isoproterenol Promotes Rapid Ryanodine Receptor Movement to Bridging Integrator 1 (BIN1)-Organized Dyads
Abstract
Background: The key pathophysiology of human acquired heart failure is impaired calcium transient, which is initiated at dyads consisting of ryanodine receptors (RyRs) at sarcoplasmic reticulum apposing CaV1.2 channels at t-tubules. Sympathetic tone regulates myocardial calcium transients through β-adrenergic receptor (β-AR)-mediated phosphorylation of dyadic proteins. Phosphorylated RyRs (P-RyR) have increased calcium sensitivity and open probability, amplifying calcium transient at a cost of receptor instability. Given that bridging integrator 1 (BIN1) organizes t-tubule microfolds and facilitates CaV1.2 delivery, we explored whether β-AR-regulated RyRs are also affected by BIN1.
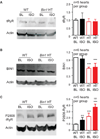
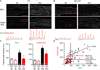
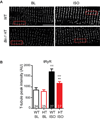
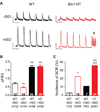
Methods and results: Isolated adult mouse hearts or cardiomyocytes were perfused for 5 minutes with the β-AR agonist isoproterenol (1 µmol/L) or the blockers CGP+ICI (baseline). Using biochemistry and superresolution fluorescent imaging, we identified that BIN1 clusters P-RyR and CaV1.2. Acute β-AR activation increases coimmunoprecipitation between P-RyR and cardiac spliced BIN1+13+17 (with exons 13 and 17). Isoproterenol redistributes BIN1 to t-tubules, recruiting P-RyRs and improving the calcium transient. In cardiac-specific Bin1 heterozygote mice, isoproterenol fails to concentrate BIN1 to t-tubules, impairing P-RyR recruitment. The resultant accumulation of uncoupled P-RyRs increases the incidence of spontaneous calcium release. In human hearts with end-stage ischemic cardiomyopathy, we find that BIN1 is also 50% reduced, with diminished P-RyR association with BIN1.
Conclusions: On β-AR activation, reorganization of BIN1-induced microdomains recruits P-RyR into dyads, increasing the calcium transient while preserving electric stability. When BIN1 is reduced as in human acquired heart failure, acute stress impairs microdomain formation, limiting contractility and promoting arrhythmias.
Keywords: BIN1 protein; heart failure; membrane microdomain; myocytes cardiac; receptors, adrenergic, beta; ryanodine receptor 2, mouse.
© 2016 American Heart Association, Inc.
Figures


References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. - PubMed
-
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. - PubMed
-
- Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation. 2001;104:688–693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

