Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease
- PMID: 26733679
- PMCID: PMC4725522
- DOI: 10.1073/pnas.1524272113
Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease
Erratum in
-
Correction for Khan et al., Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease.Proc Natl Acad Sci U S A. 2017 Jun 27;114(26):E5277. doi: 10.1073/pnas.1708559114. Epub 2017 Jun 19. Proc Natl Acad Sci U S A. 2017. PMID: 28630297 Free PMC article. No abstract available.
Abstract
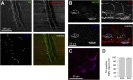
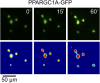
The distribution and function of sympathetic innervation in skeletal muscle have largely remained elusive. Here we demonstrate that sympathetic neurons make close contact with neuromuscular junctions and form a network in skeletal muscle that may functionally couple different targets including blood vessels, motor neurons, and muscle fibers. Direct stimulation of sympathetic neurons led to activation of muscle postsynaptic β2-adrenoreceptor (ADRB2), cAMP production, and import of the transcriptional coactivator peroxisome proliferator-activated receptor γ-coactivator 1α (PPARGC1A) into myonuclei. Electrophysiological and morphological deficits of neuromuscular junctions upon sympathectomy and in myasthenic mice were rescued by sympathicomimetic treatment. In conclusion, this study identifies the neuromuscular junction as a target of the sympathetic nervous system and shows that sympathetic input is crucial for synapse maintenance and function.
Keywords: beta-agonists; cAMP; myasthenia; neuromuscular junction; sympathetic neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


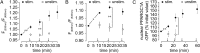





Similar articles
-
Sympathetic innervation in skeletal muscle and its role at the neuromuscular junction.J Muscle Res Cell Motil. 2024 Jun;45(2):79-86. doi: 10.1007/s10974-024-09665-9. Epub 2024 Feb 17. J Muscle Res Cell Motil. 2024. PMID: 38367152 Free PMC article. Review.
-
Postnatal Development and Distribution of Sympathetic Innervation in Mouse Skeletal Muscle.Int J Mol Sci. 2018 Jul 1;19(7):1935. doi: 10.3390/ijms19071935. Int J Mol Sci. 2018. PMID: 29966393 Free PMC article.
-
Identification of adrenergic presynaptic and postsynaptic protein locations at neuromuscular junctions, their decrease during aging, and recovery by nicotinamide mononucleotide administration.Neuroreport. 2024 Aug 7;35(12):805-812. doi: 10.1097/WNR.0000000000002070. Epub 2024 Jun 27. Neuroreport. 2024. PMID: 38935067
-
The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability.Acta Physiol (Oxf). 2019 Mar;225(3):e13195. doi: 10.1111/apha.13195. Epub 2018 Oct 22. Acta Physiol (Oxf). 2019. PMID: 30269419 Free PMC article.
-
The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia.Ageing Res Rev. 2021 May;67:101305. doi: 10.1016/j.arr.2021.101305. Epub 2021 Feb 18. Ageing Res Rev. 2021. PMID: 33610815 Free PMC article. Review.
Cited by
-
Brown to White Fat Transition Overlap With Skeletal Muscle During Development of Larger Mammals: Is it a Coincidence?J Endocr Soc. 2022 Sep 27;6(12):bvac151. doi: 10.1210/jendso/bvac151. eCollection 2022 Oct 26. J Endocr Soc. 2022. PMID: 36325536 Free PMC article. Review.
-
Adrenoceptors Modulate Cholinergic Synaptic Transmission at the Neuromuscular Junction.Int J Mol Sci. 2021 Apr 28;22(9):4611. doi: 10.3390/ijms22094611. Int J Mol Sci. 2021. PMID: 33924758 Free PMC article. Review.
-
Approach-Avoidance Decisions Under Threat: The Role of Autonomic Psychophysiological States.Front Neurosci. 2021 Mar 31;15:621517. doi: 10.3389/fnins.2021.621517. eCollection 2021. Front Neurosci. 2021. PMID: 33867915 Free PMC article.
-
Terminal Schwann Cell Aging: Implications for Age-Associated Neuromuscular Dysfunction.Aging Dis. 2021 Apr 1;12(2):494-514. doi: 10.14336/AD.2020.0708. eCollection 2021 Apr. Aging Dis. 2021. PMID: 33815879 Free PMC article. Review.
-
Dry needling in active or latent trigger point in patients with neck pain: a randomized clinical trial.Sci Rep. 2022 Feb 24;12(1):3188. doi: 10.1038/s41598-022-07063-0. Sci Rep. 2022. PMID: 35210467 Free PMC article. Clinical Trial.
References
-
- Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci. 1981;212(1188):317–332. - PubMed
-
- Silveira WA, et al. Activating cAMP/PKA signaling in skeletal muscle suppresses the ubiquitin-proteasome-dependent proteolysis: Implications for sympathetic regulation. J Appl Physiol. 2014;117(1):11–19. - PubMed
-
- Rodríguez Cruz PM, Palace J, Beeson D. Congenital myasthenic syndromes and the neuromuscular junction. Curr Opin Neurol. 2014;27(5):566–575. - PubMed
-
- Okamoto LE, Raj SR, Biaggioni I. Chronic fatigue syndrome and the autonomic nervous system. In: Robertson D, Biaggioni I, Burnstock G, Low PA, Paton JFR, editors. Primer on the Autonomic Nervous System. 3rd Ed. Academic; Amsterdam: 2012. pp. 531–534.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

