Ventral Midbrain NTS1 Receptors Mediate Conditioned Reward Induced by the Neurotensin Analog, D-Tyr[11]neurotensin
- PMID: 26733785
- PMCID: PMC4686738
- DOI: 10.3389/fnins.2015.00470
Ventral Midbrain NTS1 Receptors Mediate Conditioned Reward Induced by the Neurotensin Analog, D-Tyr[11]neurotensin
Abstract
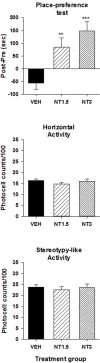
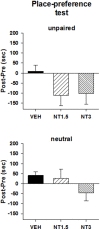
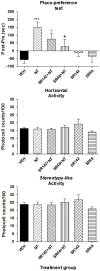
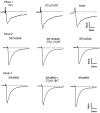
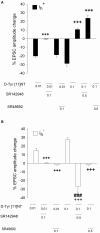
The present study was aimed at characterizing the mechanisms by which neurotensin (NT) is acting within the ventral midbrain to induce a psychostimulant-like effect. In a first experiment, we determine which subtype(s) of NT receptors is/are involved in the reward-inducing effect of ventral midbrain microinjection of NT using the conditioned place-preference (CPP) paradigm. In a second study, we used in vitro patch clamp recording technique to characterize the NT receptor subtype(s) involved in the modulation of glutamatergic neurotransmission (excitatory post-synaptic current, EPSC) in ventral tegmental neurons that expressed ([Formula: see text]), or do not express ([Formula: see text]), a hyperpolarization-activated cationic current. Behavioral studies were performed with adult male Long-Evans rats while electrophysiological recordings were obtained from brain slices of rat pups aged between 14 and 21 days. Results show that bilateral ventral midbrain microinjections of 1.5 and 3 nmol of D-Tyr[(11)]NT induced a CPP that was respectively attenuated or blocked by co-injection with 1.2 nmol of the NTS1/NTS2 antagonist, SR142948, and the preferred NTS1 antagonist, SR48692. In electrophysiological experiments, D-Tyr[(11)]NT (0.01-0.5 μM) attenuated glutamatergic EPSC in [Formula: see text] but enhanced it in [Formula: see text] neurons. The attenuation effect ([Formula: see text] neurons) was blocked by SR142948 (0.1 μM) while the enhancement effect ([Formula: see text] neurons) was blocked by both antagonists (0.1 μM). These findings suggest that (i) NT is acting on ventral midbrain NTS1 receptors to induce a rewarding effect and (ii) that this psychostimulant-like effect could be due to a direct action of NT on dopamine neurons and/or an enhancement of glutamatergic inputs to non-dopamine ([Formula: see text]) neurons.
Keywords: conditioned reward; glutamate; neurotensin; ventral midbrain.
Figures


Similar articles
-
Neurotensin enhances glutamatergic EPSCs in VTA neurons by acting on different neurotensin receptors.Peptides. 2015 Nov;73:43-50. doi: 10.1016/j.peptides.2015.08.008. Epub 2015 Aug 18. Peptides. 2015. PMID: 26296323
-
Sensitization to amphetamine psychostimulant effect: A key role for ventral tegmental area neurotensin type 2 receptors and MAP kinase pathway.Addict Biol. 2021 Sep;26(5):e13008. doi: 10.1111/adb.13008. Epub 2021 Jan 24. Addict Biol. 2021. PMID: 33491227
-
Neurotensin inhibits glutamate-mediated synaptic inputs onto ventral tegmental area dopamine neurons through the release of the endocannabinoid 2-AG.Neuropharmacology. 2012 Nov;63(6):983-91. doi: 10.1016/j.neuropharm.2012.07.037. Epub 2012 Jul 31. Neuropharmacology. 2012. PMID: 22884466
-
Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment.Prog Neurobiol. 2007 Oct;83(2):92-109. doi: 10.1016/j.pneurobio.2007.06.006. Epub 2007 Jun 28. Prog Neurobiol. 2007. PMID: 17673354 Review.
-
Relevance of dopamine D(2)/neurotensin NTS1 and NMDA/neurotensin NTS1 receptor interaction in psychiatric and neurodegenerative disorders.Curr Med Chem. 2012;19(3):304-16. doi: 10.2174/092986712803414268. Curr Med Chem. 2012. PMID: 22335510 Review.
Cited by
-
Neurotensin and Neurotensin Receptors in Stress-related Disorders: Pathophysiology & Novel Drug Targets.Curr Neuropharmacol. 2024;22(5):916-934. doi: 10.2174/1570159X21666230803101629. Curr Neuropharmacol. 2024. PMID: 37534788 Free PMC article. Review.
-
Identification of Neurotensin Receptor Expressing Cells in the Ventral Tegmental Area across the Lifespan.eNeuro. 2018 Feb 12;5(1):ENEURO.0191-17.2018. doi: 10.1523/ENEURO.0191-17.2018. eCollection 2018 Jan-Feb. eNeuro. 2018. PMID: 29464190 Free PMC article.
-
Determination of neurotensin projections to the ventral tegmental area in mice.Neuropeptides. 2018 Apr;68:57-74. doi: 10.1016/j.npep.2018.02.003. Epub 2018 Feb 15. Neuropeptides. 2018. PMID: 29478718 Free PMC article.
-
Antagonism of Neurotensin Receptors in the Ventral Tegmental Area Decreases Methamphetamine Self-Administration and Methamphetamine Seeking in Mice.Int J Neuropsychopharmacol. 2018 Apr 1;21(4):361-370. doi: 10.1093/ijnp/pyx117. Int J Neuropsychopharmacol. 2018. PMID: 29272412 Free PMC article.
-
Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance.Cell Rep. 2017 Aug 22;20(8):1881-1892. doi: 10.1016/j.celrep.2017.08.001. Cell Rep. 2017. PMID: 28834751 Free PMC article.
References
-
- al-Rodhan N. R., Richelson E., Gilbert J. A., McCormick D. J., Kanba K. S., Pfenning M. A., et al. . (1991). Structure-antinociceptive activity of neurotensin and some novel analogues in the periaqueductal gray region of the brainstem. Brain Res. 557, 227–235. - PubMed
-
- Antonelli T., Ferraro L., Fuxe K., Finetti S., Fournier J., Tanganelli S., et al. . (2004). Neurotensin enhances endogenous extracellular glutamate levels in primary cultures of rat cortical neurons: involvement of neurotensin receptor in NMDA induced excitotoxicity. Cereb. Cortex 14, 466–473. 10.1093/cercor/bhh008 - DOI - PubMed
-
- Antonelli T., Fuxe K., Tomasini M. C., Mazzoni E., Agnati L. F., Tanganelli S., et al. . (2007). Neurotensin receptor mechanisms and its modulation of glutamate transmission in the brain: relevance for neurodegenerative diseases and their treatment. Prog. Neurobiol. 83, 92–109. 10.1016/j.pneurobio.2007.06.006 - DOI - PubMed
-
- Antonelli T., Tomasini M. C., Fournier J., Mazza R., Tanganelli S., Pirondi S., et al. . (2008). Neurotensin receptor involvement in the rise of extracellular glutamate levels and apoptotic nerve cell death in primary cortical cultures after oxygen and glucose deprivation. Cereb. Cortex 18, 1748–1757. 10.1093/cercor/bhm217 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

